|
|
| (53 intermediate revisions not shown.) |
| Line 1: |
Line 1: |
| - | <StructureSection load='' size='450' side='right' scene='underdevelopment' caption=''> | + | <StructureSection load='' size='450' side='right' scene='89/896623/Cv/6' caption=''> |
| | ===Structural and catalytic characterization of ''Blastochloris viridis'' and ''Pseudomonas aeruginosa'' homospermidine synthases supports the essential role of cation-''π'' interaction=== | | ===Structural and catalytic characterization of ''Blastochloris viridis'' and ''Pseudomonas aeruginosa'' homospermidine synthases supports the essential role of cation-''π'' interaction=== |
| | <big>F. Helfrich and Axel J. Scheidig</big> <ref name="Helfrich1">PMID:34605434</ref> | | <big>F. Helfrich and Axel J. Scheidig</big> <ref name="Helfrich1">PMID:34605434</ref> |
| | <hr/> | | <hr/> |
| | <b>Molecular Tour</b><br> | | <b>Molecular Tour</b><br> |
| - | Bacterial Homospermidine Synthase | + | '''Bacterial Homospermidine Synthase''' |
| | | | |
| - | The highly conserved bacterial homospermidine synthase (HSS) is a key enzyme of the polyamine metabolism of many proteobacteria including pathogenic strains such as ''Legionella pneumophila'', ''Brucella spp.'', and various ''Pseudomonas aeruginosa'' strains<ref name="Shaw">PMID:20194510</ref>. The enzyme HSS is required for the NAD-dependent synthesis of the polyamine homospermidine (HSP) from the diamine putrescine (PUT) (Figure 1)<ref name="Tait">PMID:437275</ref>. Recently we have determined the crystal structures of two bacterial HSS, HSS from Blastochloris viridis (BvHSS) and from Pseudomonas aeruginosa (PaHSS). BvHSS exists as a homo-dimeric enzyme in solution, whereas the PaHSS is monomeric in solution but displays the same dimeric arrangement in the crystal as BvHSS<ref name="Krossa">PMID:26776105</ref>,<ref name="Helfrich1">PMID:34605434</ref>. | + | The highly conserved bacterial homospermidine synthase (HSS) is a key enzyme of the polyamine metabolism of many proteobacteria including pathogenic strains such as ''Legionella pneumophila'', ''Brucella spp.'', and various ''Pseudomonas aeruginosa'' strains<ref name="Shaw">PMID:20194510</ref>. The enzyme HSS is required for the NAD-dependent synthesis of the polyamine homospermidine (HSP) from the diamine putrescine (PUT)<ref name="Tait">PMID:437275</ref> (see static image below). |
| - | The HSS is composed of two domains, an “NAD(P)-binding Rossmann-like domain” and an “HSS-like domain” (Figure 2). The substrate binding pocket is located between these two domains. The cofactor NAD(H) is bound as a prosthetic group in the binding pocket with its nicotinamide ring being part of the active site. An “ionic slide” (BvHSS residues D94 and E117<ref name="Krossa">PMID:26776105</ref>) was proposed to lead positively charged amine substrates from the entrance of the binding pocket into the active site. The entrance tunnel is thereby lined by a so-called “track-and-trace” loop (BvHSS residues 114-130 <ref name="Krossa">PMID:26776105</ref>). Both enzymes display structural characteristics at their active site suggesting cation-π interaction through a highly conserved tryptophan as an important contribution for the catalyzed reaction.
| + | |
| - | Polyamines
| + | |
| - | Polyamines are involved in various processes in nearly all organisms in the three domains of life<ref name="Michael">PMID:27268252</ref>. In P. aeruginosa, polyamines and polyamine-related processes were demonstrated to be involved in growth<ref name="Bitonti">PMID:6818954</ref>, biofilm formation<ref name="Cardile">PMID:27864804</ref> (Qu et al., 2016; Williams et al., 2010), susceptibility to antibiotics and exogenous polyamines (Kwon & Lu, 2007; Kwon & Lu, 2006b; Kwon & Lu, 2006a, Yao, 2012 #346) as well as expression of the type III secretion system, a major virulence determinant (Anantharajah et al., 2016; Wu et al., 2012; Zhou et al., 2007). Therefore, enzymes like HSS might be promising targets for new antibiotics.
| + | |
| - | Proposed reaction mechanism of bacterial HSS
| + | |
| - | Based on crystal structures of the BvHSS, including the wildtype enzyme and several single-residue variants, a reaction mechanism depending on certain residues and the stably bound cofactor NAD(H) was proposed<ref name="Krossa">PMID:26776105</ref>. Acidic residues were suggested to attract and guide the substrate PUT via its positively charged amino groups into the binding pocket of the enzyme and to stabilize the substrate at the active site. The proposed reaction mechanism can be simplified and subdivided into two major parts as follows. First, one terminal carbon atom (atom C4) of PUT is oxidized by NAD+, forming NADH and an imine (step (1) to (3)). The imine is subsequently deaminated by nucleophilic attack of a water molecule, which yields a 4-aminobutanal (step (4)). The second part comprises another nucleophilic attack at atom C4 by the amino group of another PUT molecule (step (5/6)), yielding a Schiff base (step (7)). HSP is finally produced by electron transfer from NADH to the Schiff base, regenerating the oxidized NAD+ cofactor (step (8)). Based on the interaction geometry at the active site between the side chain of a conserved tryptophan residue and (I) the positively charged ammonium group as well as (II) the C4 atom of bound PUT and HSP molecules, cation-π interaction was suggested. In the course of the reaction, a positive charge is delocalized between carbon atom C4 and nitrogen atom N5. This charge is energetically stabilized by the π-electron system of the neighbouring indole ring of Trp229 (numbering based on BvHSS). A geometric analysis
| + | |
| - | of the positioning of the indole ring and the substrates suggests that the formation of a positive partial charge on C4 is energetically favoured and therefore stabilized.
| + | |
| - | Overall, the indole ring of residue BvHSS-Trp229 (correspondingly PaHSS-Trp225) is proposed to be involved in the stabilization of transient carbocations and positively charged nitrogen atoms (e.g. protonated imine (step 3) and the protonated Schiff base (step 7)) via cation-π interactions, thus playing a major role in lowering the transition state energy, intermediate stabilization and conversion of reaction components. Significantly reduced activity could only be retained by replacement of the tryptophan residue by the aromatic residues phenylalanine or tyrosine, supporting the requirement for an aromatic system as cation-π-interaction partner.
| + | |
| - | Figure 1. Main reaction catalyzed by HSS. Two putrescine (PUT) molecules are converted into one sym-homospermidine (HSP) molecule.
| + | |
| - | Figure 2. Cartoon representation of the dimeric PaHSS as observed in the crystal structure (PDB ID: 6Y87). The domain 1 (“NAD(P)-binding Rossmann-like”) is colored in yellow (subunit A)/orange (subunit B) and domain 2 (“homospermidine synthase (HSS)-like”) in blue (subunit A)/dark blue (subunit B). The solvent-accessible surface of the binding pocket for subunit A is depicted in transparent red with the entrance pointing upwards. The NAD+ molecule lining the surface of the pocket is shown as ball-and-stick representation.
| + | |
| - | Figure 3. Proposed reaction steps of the conversion of PUT to HSP by the bacterial HSS. Relevant residues, NAD(H), PUT, HSP and intermediates are shown as two-dimensional structure representations. Hydrogen bonds are depicted as blue dotted lines, delocalized electrons as dashed lines, cation-π interactions as orange dash-dotted lines and electron transfers as red arrows. Atom numbering is given for PUT and HSP in green. For simplicity, steps 5 and 6 are shown in combined depictions with correspondingly labeled electron transfers. A more detailed sequence of reaction steps was described before<ref name="Krossa">PMID:26776105</ref> and additional intervening reaction steps are proposed in Fig. S2 of Helfrich & Scheidig, 2021<ref name="Helfrich1">PMID:34605434</ref>.
| + | |
| - | Figure 4. Geometry of cation-π interaction between the PUT atoms C4 and N2 and the tryptophan benzene moiety in BvHSS (panel A and B, PDB ID 4TVB chain B<ref name="Krossa">PMID:26776105</ref>) and PaHSS (panel C and D, PDB ID 6Y87 chain E<ref name="Helfrich1">PMID:34605434</ref>). Structures are given as ball-and-stick representation, distances as yellow dashed lines, angle legs as grey lines and angles as grey transparent triangles (not visible for φ in (A)). The orthogonal projections of C4 and N2 onto the ring planes are shown as black spheres (C4’ and N2’). All angle legs originate from the centroid of the benzene moiety, including the dashed distance vectors (centroid to C4 and N2). Angle θ is spanned by the normal of the ring plane (grey, infinitely pointing upwards) and the C4 or N2 distance vector (yellow, dashed). Angle φ is between the vector pointing to C4’ or N2’ and the vector pointing to ring carbon CH2.
| + | |
| - | References
| + | |
| - | Anantharajah, A., Mingeot-Leclercq, M.-P. & van Bambeke, F. (2016). Trends Pharmacol. Sci. 37, 734–749.
| + | |
| | | | |
| | + | [[Image:Figure1pahss.png|left|400px|thumb|Main reaction catalyzed by HSS. Two putrescine (PUT) molecules are converted into one sym-homospermidine (HSP) molecule.]] |
| | + | {{Clear}} |
| | | | |
| - | Kwon, D.-H. & Lu, C.-D. (2007). Antimicrob. Agents Chemother. 51, 2070–2077.
| + | Recently we have determined the crystal structures of two bacterial HSS, HSS from ''Blastochloris viridis'' (BvHSS) and from ''Pseudomonas aeruginosa'' (PaHSS). BvHSS exists as a homo-dimeric enzyme in solution, whereas the PaHSS is monomeric in solution but displays the same <scene name='89/896623/Cv/9'>dimeric arrangement in the crystal</scene> as BvHSS<ref name="Helfrich1">PMID:34605434</ref>,<ref name="Krossa">PMID:26776105</ref>. <scene name='89/896623/Cv/8'>Cartoon representation of the dimeric PaHSS as observed in the crystal structure</scene> (PDB ID: [[6y87]]). The domain 1 (“NAD(P)-binding Rossmann-like”) is colored in yellow (subunit A)/gold (subunit B) and domain 2 (“homospermidine synthase (HSS)-like”) in deep sky blue (subunit A)/royal blue (subunit B). |
| - | Kwon, D. H. & Lu, C.-D. (2006a). Antimicrob. Agents Chemother. 50, 1615–1622.
| + | |
| - | Kwon, D. H. & Lu, C.-D. (2006b). Antimicrob. Agents Chemother. 50, 1623–1627.
| + | |
| | | | |
| - | Qu, L., She, P., Wang, Y., Liu, F., Zhang, D., Chen, L., Luo, Z., Xu, H., Qi, Y. & Wu, Y. (2016). Microbiologyopen 5, 402–412.
| + | The <scene name='89/896623/Cv/20'>substrate binding pocket is located between these two domains</scene>. The solvent-accessible surface of the binding pocket for subunit A is depicted in transparent red. The NAD+ molecule lining the surface of the pocket is shown as ball-and-stick representation. The cofactor NAD(H) is bound as a prosthetic group in the binding pocket with its nicotinamide ring being part of the active site. An “ionic slide” (BvHSS residues D94 and E117<ref name="Krossa">PMID:26776105</ref>) was proposed to lead positively charged amine substrates from the entrance of the binding pocket into the active site. The entrance tunnel is thereby lined by a so-called “track-and-trace” loop (BvHSS residues 114-130 <ref name="Krossa">PMID:26776105</ref>). Both enzymes display structural characteristics at their active site suggesting cation-π interaction through a highly conserved tryptophan as an important contribution for the catalyzed reaction. |
| | | | |
| | + | Geometry for cation-π interaction between the PUT atoms C4 and N2 and the tryptophan benzene moiety in BvHSS and PaHSS. |
| | | | |
| - | Williams, B. J., Du, R.-H., Calcutt, M. W., Abdolrasulnia, R., Christman, B. W. & Blackwell, T. S. (2010). Mol. Microbiol. 76, 104–119.
| + | BvHSS, PDB ID [[4tvb]], chain B<ref name="Krossa">PMID:26776105</ref>: |
| - | Wu, D., Lim, S. C., Dong, Y., Wu, J., Tao, F., Zhou, L., Zhang, L.-H. & Song, H. (2012). J. Mol. Biol. 416, 697–712.
| + | *<scene name='89/896623/Cv1/12'>Interaction geometry between PUT atom C4 and the tryptophan benzene moiety</scene> |
| - | Zhou, L., Wang, J. & Zhang, L.-H. (2007). PloS one 2, e1291.
| + | *<scene name='89/896623/Cv1/14'>Interaction geometry between PUT atom N2 and the tryptophan benzene moiety</scene> |
| | + | PaHSS, PDB ID [[6y87]], chain E<ref name="Helfrich1">PMID:34605434</ref>: |
| | + | *<scene name='89/896623/Cv1/6'>Interaction geometry between PUT atom C4 and the tryptophan benzene moiety</scene>. |
| | + | *<scene name='89/896623/Cv1/10'>Interaction geometry between PUT atom N2 and the tryptophan benzene moiety</scene>. |
| | + | Structures are given as ball-and-stick representation, distances as lines, angle legs as yellow lines for θ and white for φ; angle plane is in yellow for θ and in white for φ. The orthogonal projections of C4 and N2 onto the ring planes are shown as white spheres (C4’ and N2’). All angle legs originate from the centroid of the benzene moiety, including the distance vectors (centroid to C4 and N2). Angle θ is spanned by the normal of the ring plane and the C4 or N2 distance vector. Angle φ is between the vector pointing to C4’ or N2’ and the vector pointing to ring carbon CH2. |
| | | | |
| | + | '''Polyamines''' |
| | + | |
| | + | Polyamines are involved in various processes in nearly all organisms in the three domains of life<ref name="Michael">PMID:27268252</ref>. In ''P. aeruginosa'', polyamines and polyamine-related processes were demonstrated to be involved in growth<ref name="Bitonti">PMID:6818954</ref>, biofilm formation<ref name="Cardile">PMID:27864804</ref>,<ref name="Qu">PMID:26817804</ref>,<ref name="Williams">PMID:20149107</ref>, susceptibility to antibiotics and exogenous polyamines<ref name="Kwon">PMID:17438056</ref>,<ref name="Kwonb">PMID:16641427</ref>,<ref name="Kwona">PMID:16641426</ref>,<ref name="Yao">PMID:22869561</ref> as well as expression of the type III secretion system, a major virulence determinant<ref name="Anantharajah">PMID:27344210</ref>,<ref name="Wu">PMID:22300763</ref>,<ref name="Zhou">PMID:18074016</ref>. Therefore, enzymes like HSS might be promising targets for new antibiotics. |
| | + | |
| | + | '''Proposed reaction mechanism of bacterial HSS''' |
| | + | |
| | + | [[Image:Figure3pahss3.png|left|400px|thumb|Figure 3. Proposed reaction steps of the conversion of PUT to HSP by the bacterial HSS. Relevant residues, NAD(H), PUT, HSP and intermediates are shown as two-dimensional structure representations. Hydrogen bonds are depicted as blue dotted lines, delocalized electrons as dashed lines, cation-π interactions as orange dash-dotted lines and electron transfers as red arrows. Atom numbering is given for PUT and HSP in green. For simplicity, steps 5 and 6 are shown in combined depictions with correspondingly labeled electron transfers. A more detailed sequence of reaction steps was described before<ref name="Krossa">PMID:26776105</ref> and additional intervening reaction steps are proposed in Fig. S2 of Helfrich & Scheidig, 2021<ref name="Helfrich1">PMID:34605434</ref>.]] |
| | + | {{Clear}} |
| | + | Based on crystal structures of the BvHSS, including the wildtype enzyme and several single-residue variants, a reaction mechanism depending on certain residues and the stably bound cofactor NAD(H) was proposed<ref name="Krossa">PMID:26776105</ref>. Acidic residues were suggested to attract and guide the substrate PUT via its positively charged amino groups into the binding pocket of the enzyme and to stabilize the substrate at the active site. The proposed reaction mechanism can be simplified and subdivided into two major parts as follows. First, one terminal carbon atom (atom C4) of PUT is oxidized by NAD+, forming NADH and an imine (step (1) to (3)). The imine is subsequently deaminated by nucleophilic attack of a water molecule, which yields a 4-aminobutanal (step (4)). The second part comprises another nucleophilic attack at atom C4 by the amino group of another PUT molecule (step (5/6)), yielding a Schiff base (step (7)). HSP is finally produced by electron transfer from NADH to the Schiff base, regenerating the oxidized NAD+ cofactor (step (8)). Based on the interaction geometry at the active site between the side chain of a conserved tryptophan residue and (I) the positively charged ammonium group as well as (II) the C4 atom of bound PUT and HSP molecules, cation-π interaction was suggested. In the course of the reaction, a positive charge is delocalized between carbon atom C4 and nitrogen atom N5. This charge is energetically stabilized by the π-electron system of the neighbouring indole ring of Trp229 (numbering based on BvHSS). A geometric analysis of the positioning of the indole ring and the substrates suggests that the formation of a positive partial charge on C4 is energetically favoured and therefore stabilized. |
| | + | |
| | + | Overall, the indole ring of residue BvHSS-Trp229 (correspondingly PaHSS-Trp225) is proposed to be involved in the stabilization of transient carbocations and positively charged nitrogen atoms (''e.g.'' protonated imine (step 3) and the protonated Schiff base (step 7)) via cation-π interactions, thus playing a major role in lowering the transition state energy, intermediate stabilization and conversion of reaction components. Significantly reduced activity could only be retained by replacement of the tryptophan residue by the aromatic residues phenylalanine or tyrosine, supporting the requirement for an aromatic system as cation-π-interaction partner. |
| | + | |
| | + | '''PDB references:''' homospermidine synthase from ''Blastochloris viridis'', W229E variant, complex with NAD, [[6sep]]; E210Q variant, complex with NAD, [[6s3x]]; W229F variant, complex with NAD, [[6s4d]]; E210A variant, complex with NAD, [[6s49]]; E117Q variant, complex with NAD, [[6s6g]]; W229A variant, complex with NAD and PUT, [[6s72]]; from ''Pseudomonas aeruginosa'', complex with NAD and PUT, [[6y87]]. |
| | | | |
| | <b>References</b><br> | | <b>References</b><br> |
| Structural and catalytic characterization of Blastochloris viridis and Pseudomonas aeruginosa homospermidine synthases supports the essential role of cation-π interaction
F. Helfrich and Axel J. Scheidig [1]
Molecular Tour
Bacterial Homospermidine Synthase
The highly conserved bacterial homospermidine synthase (HSS) is a key enzyme of the polyamine metabolism of many proteobacteria including pathogenic strains such as Legionella pneumophila, Brucella spp., and various Pseudomonas aeruginosa strains[2]. The enzyme HSS is required for the NAD-dependent synthesis of the polyamine homospermidine (HSP) from the diamine putrescine (PUT)[3] (see static image below).
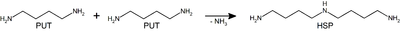 Main reaction catalyzed by HSS. Two putrescine (PUT) molecules are converted into one sym-homospermidine (HSP) molecule. Recently we have determined the crystal structures of two bacterial HSS, HSS from Blastochloris viridis (BvHSS) and from Pseudomonas aeruginosa (PaHSS). BvHSS exists as a homo-dimeric enzyme in solution, whereas the PaHSS is monomeric in solution but displays the same as BvHSS[1],[4]. (PDB ID: 6y87). The domain 1 (“NAD(P)-binding Rossmann-like”) is colored in yellow (subunit A)/gold (subunit B) and domain 2 (“homospermidine synthase (HSS)-like”) in deep sky blue (subunit A)/royal blue (subunit B).
The . The solvent-accessible surface of the binding pocket for subunit A is depicted in transparent red. The NAD+ molecule lining the surface of the pocket is shown as ball-and-stick representation. The cofactor NAD(H) is bound as a prosthetic group in the binding pocket with its nicotinamide ring being part of the active site. An “ionic slide” (BvHSS residues D94 and E117[4]) was proposed to lead positively charged amine substrates from the entrance of the binding pocket into the active site. The entrance tunnel is thereby lined by a so-called “track-and-trace” loop (BvHSS residues 114-130 [4]). Both enzymes display structural characteristics at their active site suggesting cation-π interaction through a highly conserved tryptophan as an important contribution for the catalyzed reaction.
Geometry for cation-π interaction between the PUT atoms C4 and N2 and the tryptophan benzene moiety in BvHSS and PaHSS.
BvHSS, PDB ID 4tvb, chain B[4]:
PaHSS, PDB ID 6y87, chain E[1]:
Structures are given as ball-and-stick representation, distances as lines, angle legs as yellow lines for θ and white for φ; angle plane is in yellow for θ and in white for φ. The orthogonal projections of C4 and N2 onto the ring planes are shown as white spheres (C4’ and N2’). All angle legs originate from the centroid of the benzene moiety, including the distance vectors (centroid to C4 and N2). Angle θ is spanned by the normal of the ring plane and the C4 or N2 distance vector. Angle φ is between the vector pointing to C4’ or N2’ and the vector pointing to ring carbon CH2.
Polyamines
Polyamines are involved in various processes in nearly all organisms in the three domains of life[5]. In P. aeruginosa, polyamines and polyamine-related processes were demonstrated to be involved in growth[6], biofilm formation[7],[8],[9], susceptibility to antibiotics and exogenous polyamines[10],[11],[12],[13] as well as expression of the type III secretion system, a major virulence determinant[14],[15],[16]. Therefore, enzymes like HSS might be promising targets for new antibiotics.
Proposed reaction mechanism of bacterial HSS
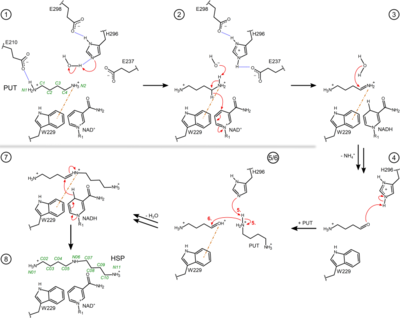 Figure 3. Proposed reaction steps of the conversion of PUT to HSP by the bacterial HSS. Relevant residues, NAD(H), PUT, HSP and intermediates are shown as two-dimensional structure representations. Hydrogen bonds are depicted as blue dotted lines, delocalized electrons as dashed lines, cation-π interactions as orange dash-dotted lines and electron transfers as red arrows. Atom numbering is given for PUT and HSP in green. For simplicity, steps 5 and 6 are shown in combined depictions with correspondingly labeled electron transfers. A more detailed sequence of reaction steps was described before [4] and additional intervening reaction steps are proposed in Fig. S2 of Helfrich & Scheidig, 2021 [1]. Based on crystal structures of the BvHSS, including the wildtype enzyme and several single-residue variants, a reaction mechanism depending on certain residues and the stably bound cofactor NAD(H) was proposed[4]. Acidic residues were suggested to attract and guide the substrate PUT via its positively charged amino groups into the binding pocket of the enzyme and to stabilize the substrate at the active site. The proposed reaction mechanism can be simplified and subdivided into two major parts as follows. First, one terminal carbon atom (atom C4) of PUT is oxidized by NAD+, forming NADH and an imine (step (1) to (3)). The imine is subsequently deaminated by nucleophilic attack of a water molecule, which yields a 4-aminobutanal (step (4)). The second part comprises another nucleophilic attack at atom C4 by the amino group of another PUT molecule (step (5/6)), yielding a Schiff base (step (7)). HSP is finally produced by electron transfer from NADH to the Schiff base, regenerating the oxidized NAD+ cofactor (step (8)). Based on the interaction geometry at the active site between the side chain of a conserved tryptophan residue and (I) the positively charged ammonium group as well as (II) the C4 atom of bound PUT and HSP molecules, cation-π interaction was suggested. In the course of the reaction, a positive charge is delocalized between carbon atom C4 and nitrogen atom N5. This charge is energetically stabilized by the π-electron system of the neighbouring indole ring of Trp229 (numbering based on BvHSS). A geometric analysis of the positioning of the indole ring and the substrates suggests that the formation of a positive partial charge on C4 is energetically favoured and therefore stabilized.
Overall, the indole ring of residue BvHSS-Trp229 (correspondingly PaHSS-Trp225) is proposed to be involved in the stabilization of transient carbocations and positively charged nitrogen atoms (e.g. protonated imine (step 3) and the protonated Schiff base (step 7)) via cation-π interactions, thus playing a major role in lowering the transition state energy, intermediate stabilization and conversion of reaction components. Significantly reduced activity could only be retained by replacement of the tryptophan residue by the aromatic residues phenylalanine or tyrosine, supporting the requirement for an aromatic system as cation-π-interaction partner.
PDB references: homospermidine synthase from Blastochloris viridis, W229E variant, complex with NAD, 6sep; E210Q variant, complex with NAD, 6s3x; W229F variant, complex with NAD, 6s4d; E210A variant, complex with NAD, 6s49; E117Q variant, complex with NAD, 6s6g; W229A variant, complex with NAD and PUT, 6s72; from Pseudomonas aeruginosa, complex with NAD and PUT, 6y87.
References
- ↑ 1.0 1.1 1.2 1.3 Helfrich F, Scheidig AJ. Structural and catalytic characterization of Blastochloris viridis and Pseudomonas aeruginosa homospermidine synthases supports the essential role of cation-pi interaction. Acta Crystallogr D Struct Biol. 2021 Oct 1;77(Pt 10):1317-1335. doi:, 10.1107/S2059798321008937. Epub 2021 Sep 23. PMID:34605434 doi:http://dx.doi.org/10.1107/S2059798321008937
- ↑ Shaw FL, Elliott KA, Kinch LN, Fuell C, Phillips MA, Michael AJ. Evolution and multifarious horizontal transfer of an alternative biosynthetic pathway for the alternative polyamine sym-homospermidine. J Biol Chem. 2010 May 7;285(19):14711-23. doi: 10.1074/jbc.M110.107219. Epub 2010, Mar 1. PMID:20194510 doi:http://dx.doi.org/10.1074/jbc.M110.107219
- ↑ Tait GH. The formation of homospermidine by an enzyme from Rhodopseudomonas viridis [proceedings]. Biochem Soc Trans. 1979 Feb;7(1):199-201. doi: 10.1042/bst0070199. PMID:437275 doi:http://dx.doi.org/10.1042/bst0070199
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Krossa S, Faust A, Ober D, Scheidig AJ. Comprehensive Structural Characterization of the Bacterial Homospermidine Synthase-an Essential Enzyme of the Polyamine Metabolism. Sci Rep. 2016 Jan 18;6:19501. doi: 10.1038/srep19501. PMID:26776105 doi:http://dx.doi.org/10.1038/srep19501
- ↑ Michael AJ. Polyamines in Eukaryotes, Bacteria, and Archaea. J Biol Chem. 2016 Jul 15;291(29):14896-903. doi: 10.1074/jbc.R116.734780. Epub, 2016 Jun 7. PMID:27268252 doi:http://dx.doi.org/10.1074/jbc.R116.734780
- ↑ Bitonti AJ, McCann PP, Sjoerdsma A. Restriction of bacterial growth by inhibition of polyamine biosynthesis by using monofluoromethylornithine, difluoromethylarginine and dicyclohexylammonium sulphate. Biochem J. 1982 Nov 15;208(2):435-41. doi: 10.1042/bj2080435. PMID:6818954 doi:http://dx.doi.org/10.1042/bj2080435
- ↑ Cardile AP, Woodbury RL, Sanchez CJ Jr, Becerra SC, Garcia RA, Mende K, Wenke JC, Akers KS. Activity of Norspermidine on Bacterial Biofilms of Multidrug-Resistant Clinical Isolates Associated with Persistent Extremity Wound Infections. Adv Exp Med Biol. 2017;973:53-70. doi: 10.1007/5584_2016_93. PMID:27864804 doi:http://dx.doi.org/10.1007/5584_2016_93
- ↑ Qu L, She P, Wang Y, Liu F, Zhang D, Chen L, Luo Z, Xu H, Qi Y, Wu Y. Effects of norspermidine on Pseudomonas aeruginosa biofilm formation and eradication. Microbiologyopen. 2016 Jun;5(3):402-12. doi: 10.1002/mbo3.338. Epub 2016 Jan 27. PMID:26817804 doi:http://dx.doi.org/10.1002/mbo3.338
- ↑ Williams BJ, Du RH, Calcutt MW, Abdolrasulnia R, Christman BW, Blackwell TS. Discovery of an operon that participates in agmatine metabolism and regulates biofilm formation in Pseudomonas aeruginosa. Mol Microbiol. 2010 Apr;76(1):104-19. doi: 10.1111/j.1365-2958.2010.07083.x. Epub, 2010 Feb 10. PMID:20149107 doi:http://dx.doi.org/10.1111/j.1365-2958.2010.07083.x
- ↑ Kwon DH, Lu CD. Polyamine effects on antibiotic susceptibility in bacteria. Antimicrob Agents Chemother. 2007 Jun;51(6):2070-7. doi: 10.1128/AAC.01472-06., Epub 2007 Apr 16. PMID:17438056 doi:http://dx.doi.org/10.1128/AAC.01472-06
- ↑ Kwon DH, Lu CD. Polyamines increase antibiotic susceptibility in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006 May;50(5):1623-7. doi:, 10.1128/AAC.50.5.1623-1627.2006. PMID:16641427 doi:http://dx.doi.org/10.1128/AAC.50.5.1623-1627.2006
- ↑ Kwon DH, Lu CD. Polyamines induce resistance to cationic peptide, aminoglycoside, and quinolone antibiotics in Pseudomonas aeruginosa PAO1. Antimicrob Agents Chemother. 2006 May;50(5):1615-22. doi:, 10.1128/AAC.50.5.1615-1622.2006. PMID:16641426 doi:http://dx.doi.org/10.1128/AAC.50.5.1615-1622.2006
- ↑ Yao X, Li C, Zhang J, Lu CD. gamma-glutamyl Spermine Synthetase PauA2 as a potential target of antibiotic development against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2012 Oct;56(10):5309-14. doi: 10.1128/AAC.01158-12. , Epub 2012 Aug 6. PMID:22869561 doi:http://dx.doi.org/10.1128/AAC.01158-12
- ↑ Anantharajah A, Mingeot-Leclercq MP, Van Bambeke F. Targeting the Type Three Secretion System in Pseudomonas aeruginosa. Trends Pharmacol Sci. 2016 Sep;37(9):734-749. doi: 10.1016/j.tips.2016.05.011., Epub 2016 Jun 22. PMID:27344210 doi:http://dx.doi.org/10.1016/j.tips.2016.05.011
- ↑ Wu D, Lim SC, Dong Y, Wu J, Tao F, Zhou L, Zhang LH, Song H. Structural Basis of Substrate Binding Specificity Revealed by the Crystal Structures of Polyamine Receptors SpuD and SpuE from Pseudomonas aeruginosa. J Mol Biol. 2012 Mar 9;416(5):697-712. Epub 2012 Jan 28. PMID:22300763 doi:10.1016/j.jmb.2012.01.010
- ↑ Zhou L, Wang J, Zhang LH. Modulation of bacterial Type III secretion system by a spermidine transporter dependent signaling pathway. PLoS One. 2007 Dec 12;2(12):e1291. doi: 10.1371/journal.pone.0001291. PMID:18074016 doi:http://dx.doi.org/10.1371/journal.pone.0001291
|



