This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1660
From Proteopedia
(Difference between revisions)
| (10 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
{{Sandbox_Reserved_ESBS20_}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_Reserved_ESBS20_}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| - | <StructureSection load='3GMQ' size='350' 'frame'='true' side='right' caption='Structure of mouse CD1d | + | <StructureSection load='3GMQ' size='350' 'frame'='true' side='right' caption='Structure of mouse CD1d (PDB entry : [https://www.rcsb.org/structure/3GMQ 3GMQ])' scene='86/868193/Cd1d/7'> |
==''' The CD1 family'''== | ==''' The CD1 family'''== | ||
<p align="justify"> | <p align="justify"> | ||
| - | '''[[CD1]] (Cluster of Differentiation 1)''' is a family of glycoproteins involved in the presentation of antigens on the surface of specific cells to [https://en.wikipedia.org/wiki/Natural_killer_T_cell NKT cells]. CD1 molecules are notably expressed by splenic dendritic cells, marginal zone B cells and CD4+CD8+ thymocytes. The CD1 family is made of three groups. Group 1 is composed of CD1a, b and c proteins and group 2 is composed of CD1d proteins. The third group is the CD1e, which shares some partial characteristics from groups 1 and 2<ref>Jullien, D.; Afanassieff, M.; Claudy, A.; Nicolas, J.; Kaiserlian, D. CD1 : une nouvelle famille de molécules présentatrices d’antigènes aux caractéristiques singulières. Med Sci (Paris) 1999, 15 (1), 7. https://doi.org/10.4267/10608/1190.</ref> <ref>Angenieux C, Salamero J, Fricker D, Cazenave JP, Goud B, Hanau D, de La Salle H. Characterization of CD1e, a third type of CD1 molecule expressed in dendritic cells. J Biol Chem. 2000 Dec 1;275(48):37757-64. http://doi.org/10.1074/jbc.M007082200 | + | '''[[CD1]] (Cluster of Differentiation 1)''' is a family of glycoproteins involved in the presentation of antigens on the surface of specific cells to [https://en.wikipedia.org/wiki/Natural_killer_T_cell NKT cells]. CD1 molecules are notably expressed by splenic dendritic cells, marginal zone B cells and CD4+CD8+ thymocytes. The CD1 family is made of three groups. Group 1 is composed of CD1a, b and c proteins and group 2 is composed of CD1d proteins. The third group is the CD1e, which shares some partial characteristics from groups 1 and 2<ref>Jullien, D.; Afanassieff, M.; Claudy, A.; Nicolas, J.; Kaiserlian, D. CD1 : une nouvelle famille de molécules présentatrices d’antigènes aux caractéristiques singulières. Med Sci (Paris) 1999, 15 (1), 7. https://doi.org/10.4267/10608/1190.</ref> <ref>Angenieux C, Salamero J, Fricker D, Cazenave JP, Goud B, Hanau D, de La Salle H. Characterization of CD1e, a third type of CD1 molecule expressed in dendritic cells. J Biol Chem. 2000 Dec 1;275(48):37757-64. http://doi.org/10.1074/jbc.M007082200 PMID: 10948205.</ref>. |
<br> | <br> | ||
Thus, the structure and function of such proteins in mice are akin to those of humans. Mice do not express group 1 CD1 molecules, but they have two kinds of CD1d molecules. Therefore, mice have been widely used to characterize the functions of CD1d and CD1d-dependent NKT cells in many diseases. | Thus, the structure and function of such proteins in mice are akin to those of humans. Mice do not express group 1 CD1 molecules, but they have two kinds of CD1d molecules. Therefore, mice have been widely used to characterize the functions of CD1d and CD1d-dependent NKT cells in many diseases. | ||
| Line 13: | Line 13: | ||
__TOC__ | __TOC__ | ||
| - | == Function == | + | =='''Function'''== |
[[Image:CD1d dependent lipid antigen presentation.jpg | thumb ||left|190px|'''CD1d dependent lipid antigen presentation.''']] | [[Image:CD1d dependent lipid antigen presentation.jpg | thumb ||left|190px|'''CD1d dependent lipid antigen presentation.''']] | ||
| Line 22: | Line 22: | ||
'''CD1d protein''' is a molecule of the [https://en.wikipedia.org/wiki/Immune_system immune system], involved in the presentation of a lipid antigen to NKT cells, which are a subset of [https://en.wikipedia.org/wiki/T_cell T cells]. Indeed, these proteins are located on the plasma membrane of [https://en.wikipedia.org/wiki/Antigen-presenting_cell APC cells]. When the recognition between the CD1d bound to its lipid ligand and the [[TCR]] of a NKT cell occurs, the [https://en.wikipedia.org/wiki/Lymphocyte lymphocyte] turns out to be activated. Thus, the production of cytotoxic molecules such as [[interleukin]]-4 and [https://en.wikipedia.org/wiki/Interferon_gamma IFN-gamma] is triggered by this activation, leading to a Th2 or Th1 immune response respectively<ref name="properties">Joyce, S. CD1d and Natural T Cells: How Their Properties Jump-Start the Immune System. CMLS, Cell. Mol. Life Sci. 2001, 58 (3), 442–469. https://doi.org/10.1007/PL00000869.</ref><ref name="affinity">Rossjohn, J., Pellicci, D. G., Patel, O., Gapin, L., & Godfrey, D. I. (2012). Recognition of CD1d-restricted antigens by natural killer T cells. Nature reviews. Immunology, 12(12), 845–857. https://doi.org/10.1038/nri3328.</ref>. Therefore, CD1d proteins are precursors of the adaptive immune reaction. As a result, a deficiency of CD1d protein may lead to a deficiency of the NKT cells functioning and thus to [https://en.wikipedia.org/wiki/Autoimmune_disease autoimmune diseases] and [https://en.wikipedia.org/wiki/Cancer cancers]. | '''CD1d protein''' is a molecule of the [https://en.wikipedia.org/wiki/Immune_system immune system], involved in the presentation of a lipid antigen to NKT cells, which are a subset of [https://en.wikipedia.org/wiki/T_cell T cells]. Indeed, these proteins are located on the plasma membrane of [https://en.wikipedia.org/wiki/Antigen-presenting_cell APC cells]. When the recognition between the CD1d bound to its lipid ligand and the [[TCR]] of a NKT cell occurs, the [https://en.wikipedia.org/wiki/Lymphocyte lymphocyte] turns out to be activated. Thus, the production of cytotoxic molecules such as [[interleukin]]-4 and [https://en.wikipedia.org/wiki/Interferon_gamma IFN-gamma] is triggered by this activation, leading to a Th2 or Th1 immune response respectively<ref name="properties">Joyce, S. CD1d and Natural T Cells: How Their Properties Jump-Start the Immune System. CMLS, Cell. Mol. Life Sci. 2001, 58 (3), 442–469. https://doi.org/10.1007/PL00000869.</ref><ref name="affinity">Rossjohn, J., Pellicci, D. G., Patel, O., Gapin, L., & Godfrey, D. I. (2012). Recognition of CD1d-restricted antigens by natural killer T cells. Nature reviews. Immunology, 12(12), 845–857. https://doi.org/10.1038/nri3328.</ref>. Therefore, CD1d proteins are precursors of the adaptive immune reaction. As a result, a deficiency of CD1d protein may lead to a deficiency of the NKT cells functioning and thus to [https://en.wikipedia.org/wiki/Autoimmune_disease autoimmune diseases] and [https://en.wikipedia.org/wiki/Cancer cancers]. | ||
</p> | </p> | ||
| - | <br> | ||
==== Ligands presented by CD1d ==== | ==== Ligands presented by CD1d ==== | ||
<p align="justify"> | <p align="justify"> | ||
| - | The ligands that can be presented by CD1d to NKT or other CD1d-restricted T cells are quite specific, because the recognition is based on a hydrophobic interaction between the ligand and the CD1d molecule. Thus, it limits the risk | + | The ligands that can be presented by CD1d to NKT or other CD1d-restricted T cells are quite specific, because the recognition is based on a hydrophobic interaction between the ligand and the CD1d molecule. Thus, it limits the risk for other similar molecules to bind CD1d proteins and ensures that the immune response is accurate. Among these CD1d ligands, glycolipids from a marine sponge (alphagalactosylceramide or α-GalCer), bacterial glycolipids, normal endogenous glycolipids, tumor-derived phospholipids and glycolipids, and nonlipidic molecules have been described<ref name="Immunity">Brutkiewicz, R. R. CD1d Ligands: The Good, the Bad, and the Ugly. The Journal of Immunology 2006, 177 (2), 769–775. https://doi.org/10.4049/jimmunol.177.2.769.</ref>. |
</p> | </p> | ||
<br> | <br> | ||
| - | == Structure of mouse CD1d == | + | =='''Structure of mouse CD1d'''== |
<p align="justify"> | <p align="justify"> | ||
| - | <scene name='86/868193/Cd1d/ | + | <scene name='86/868193/Cd1d/7'>CD1d protein</scene> is made of 2 chains <ref name="Structure">Information, N. C. for B.; Pike, U. S. N. L. of M. R.; BethesdaMD; 20894USA. 3GMQ: Structure of mouse CD1d expressed in SF9 cells, no ligand added https://www.ncbi.nlm.nih.gov/Structure/pdb/3GMQ (accessed Dec 5, 2020).</ref>: <br> |
:- an <scene name='86/868193/Alpha_chain/9'>alpha chain</scene> (T-cell surface glycoprotein CD1d1) of 287 amino acids <br> | :- an <scene name='86/868193/Alpha_chain/9'>alpha chain</scene> (T-cell surface glycoprotein CD1d1) of 287 amino acids <br> | ||
:- a <scene name='86/868193/Beta-2-microglobulin_chain/7'>beta-2-microglobulin chain</scene> of 99 amino acids. <br> | :- a <scene name='86/868193/Beta-2-microglobulin_chain/7'>beta-2-microglobulin chain</scene> of 99 amino acids. <br> | ||
The alpha chain is made of three domains: alpha 1, alpha 2 and alpha 3. The association of alpha 1 and alpha 2 is composed of two [https://en.wikipedia.org/wiki/Beta_sheet beta-sheets] and a set of 2 [https://proteopedia.org/wiki/index.php/Alpha_helix alpha helixes]. Each beta-sheet contains four antiparallel strands. The ligand binds between the two alpha 1 and 2 helices. The alpha 3 domain is non-covalently bound with beta-2-microglobulin domain. | The alpha chain is made of three domains: alpha 1, alpha 2 and alpha 3. The association of alpha 1 and alpha 2 is composed of two [https://en.wikipedia.org/wiki/Beta_sheet beta-sheets] and a set of 2 [https://proteopedia.org/wiki/index.php/Alpha_helix alpha helixes]. Each beta-sheet contains four antiparallel strands. The ligand binds between the two alpha 1 and 2 helices. The alpha 3 domain is non-covalently bound with beta-2-microglobulin domain. | ||
| - | Additionally, there are five <scene name='86/868193/Oligosaccharides/ | + | Additionally, there are five <scene name='86/868193/Oligosaccharides/4'>oligosaccharides</scene><ref name="Structure"/> bound to the alpha chain via N-glycosylations, three of which have been clearly identified<ref name="oligo">Sriram, V., Willard, C.A., Liu, J., & Brutkiewicz, R.R.(2008). Importance of N-linked glycosylation in the functional expression of murine CD1d1. Immunology, 123:272–281.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2433293/</ref>. The total molecular weight of the alpha chain is 33 kDa when not associated to any oligosaccharide and 55 kDa when oligosaccharides are associated to the chain<ref name="properties"/>.</p> |
| - | CD1d molecules are structurally similar to [[Major Histocompatibility Complex Class I]], but present lipid antigens as opposed to peptides. Thus the cleft where the ligand can bind is different between MHC molecules and CD1d molecules. Indeed, the hydrophobic cleft of CD1d has a narrow opening.The recognition between the protein and its ligand occurs at a specific hydrophobic spot which creates an appropriate environment for the interaction to happen. This <scene name='86/868193/Site/ | + | CD1d molecules are structurally similar to [[Major Histocompatibility Complex Class I]], but present lipid antigens as opposed to peptides. Thus the cleft where the ligand can bind is different between MHC molecules and CD1d molecules. Indeed, the hydrophobic cleft of CD1d has a narrow opening.The recognition between the protein and its ligand occurs at a specific hydrophobic spot which creates an appropriate environment for the interaction to happen. This <scene name='86/868193/Site/7'>site</scene> is located at the A' and F' pockets in the region of the alpha helices<ref name="site">Schiefner, A.; Fujio, M.; Wu, D.; Wong, C.-H.; Wilson, I. A. Structural Evaluation of Potent NKT-Cell Agonists: Implications for Design of Novel Stimulatory Ligands. J Mol Biol 2009, 394 (1), 71–82. https://doi.org/10.1016/j.jmb.2009.08.061</ref>. |
| - | < | + | <br><br> |
| - | + | =='''Impact of ligand-binding'''== | |
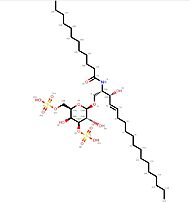
| - | == Impact of ligand-binding == | + | [[Image:C12DS.jpg | thumb ||left|190px|'''Ligand C12-di-sulfatide''']] |
| - | + | ||
==== Conformational variation ==== | ==== Conformational variation ==== | ||
| - | + | The stability of the CD1d-glycolipid complexes has an impact on the [https://en.wikipedia.org/wiki/Cytokine cytokine] release (cell signaling). Conformational variations that would stabilize the F’-pocket (primary site of interaction with the T cell receptor, NKT TCR) might increase CD1d affinity for the NKT TCR<ref name="affinity"/><ref name="site"/>. | |
| - | + | ||
| - | The stability of the CD1d-glycolipid complexes has an impact on the [https://en.wikipedia.org/wiki/Cytokine cytokine] release (cell signaling). Conformational variations that would stabilize the F’-pocket (primary site of interaction with the T cell receptor, NKT TCR) might increase CD1d affinity for the NKT TCR<ref name="affinity"/><ref name="site"/>. | + | |
| - | + | ||
The binding of a ligand (such as C12-di-sulfatide) leads to <scene name='86/868193/Conformational_variations/1'>conformational variations</scene> of CD1d molecule (The moving molecules are the oligosaccharides molecules ; the ligand is not represented). | The binding of a ligand (such as C12-di-sulfatide) leads to <scene name='86/868193/Conformational_variations/1'>conformational variations</scene> of CD1d molecule (The moving molecules are the oligosaccharides molecules ; the ligand is not represented). | ||
| Line 56: | Line 51: | ||
<p align="justify"> | <p align="justify"> | ||
CD1d proteins lipid recognition is based on the interaction of the protein with its ligand. Nevertheless, the interaction relies on two recognitions. The first one is the recognition of the head of the lipid and the second one is the recognition of the length of the molecule. Because there are more than one condition to fill in order to interact with CD1d proteins, the affinity for a lipid depends itself on a plurality of parameters which modulates it<ref name="affinity"/><ref name="site"/><ref>McCarthy, C.; Shepherd, D.; Fleire, S.; Stronge, V. S.; Koch, M.; Illarionov, P. A.; Bossi, G.; Salio, M.; Denkberg, G.; Reddington, F.; Tarlton, A.; Reddy, B. G.; Schmidt, R. R.; Reiter, Y.; Griffiths, G. M.; van der Merwe, P. A.; Besra, G. S.; Jones, E. Y.; Batista, F. D.; Cerundolo, V. The Length of Lipids Bound to Human CD1d Molecules Modulates the Affinity of NKT Cell TCR and the Threshold of NKT Cell Activation. J Exp Med 2007, 204 (5), 1131–1144. https://doi.org/10.1084/jem.20062342.</ref>. | CD1d proteins lipid recognition is based on the interaction of the protein with its ligand. Nevertheless, the interaction relies on two recognitions. The first one is the recognition of the head of the lipid and the second one is the recognition of the length of the molecule. Because there are more than one condition to fill in order to interact with CD1d proteins, the affinity for a lipid depends itself on a plurality of parameters which modulates it<ref name="affinity"/><ref name="site"/><ref>McCarthy, C.; Shepherd, D.; Fleire, S.; Stronge, V. S.; Koch, M.; Illarionov, P. A.; Bossi, G.; Salio, M.; Denkberg, G.; Reddington, F.; Tarlton, A.; Reddy, B. G.; Schmidt, R. R.; Reiter, Y.; Griffiths, G. M.; van der Merwe, P. A.; Besra, G. S.; Jones, E. Y.; Batista, F. D.; Cerundolo, V. The Length of Lipids Bound to Human CD1d Molecules Modulates the Affinity of NKT Cell TCR and the Threshold of NKT Cell Activation. J Exp Med 2007, 204 (5), 1131–1144. https://doi.org/10.1084/jem.20062342.</ref>. | ||
| - | </p> | + | </p><br> |
| - | == Applications == | + | =='''Applications'''== |
==== Immunotherapeutic tool ==== | ==== Immunotherapeutic tool ==== | ||
<p align="justify"> | <p align="justify"> | ||
| - | The presentation of several | + | The presentation of several kinds of ligands can have immunopotentiating effects, such as serving as an adjuvant in [https://en.wikipedia.org/wiki/Malaria malaria] vaccine or resulting in a more rapid clearance of certain [https://en.wikipedia.org/wiki/Virus virus] infections. They can also be protective in autoimmune diseases or cancer<ref name="affinity"/><ref name="Immunity"/><ref>Sköld, M.; Behar, S. M. Role of CD1d-Restricted NKT Cells in Microbial Immunity. Infect Immun 2003, 71 (10), 5447–5455. https://doi.org/10.1128/IAI.71.10.5447-5455.2003.</ref>. |
</p> | </p> | ||
Current revision
| This Sandbox is Reserved from 26/11/2020, through 26/11/2021 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1643 through Sandbox Reserved 1664. |
To get started:
More help: Help:Editing |
| |||||||||||