Journal:BAMBEd:Acetylcholinesterase: Substrate Traffic and Inhibition
From Proteopedia
(Difference between revisions)

| (One intermediate revision not shown.) | |||
| Line 1: | Line 1: | ||
<StructureSection load='2ace' size='350' side='right' scene='Sandbox_250/Ache_ach/30' caption=''> | <StructureSection load='2ace' size='350' side='right' scene='Sandbox_250/Ache_ach/30' caption=''> | ||
| - | + | ===Acetylcholinesterase: Substrate traffic and inhibition=== | |
| + | <big>Mary G. Acheampong, Daviana E. Dueño, Bobby K. Glover, Alafia A. Henry, Randol Mata, Marisa L. VanBrakle, Lars F. Westblade, Joel L. Sussman, Allison L. Granberry</big> <ref>doi: 10.1002/bmb.20604</ref> | ||
| + | <hr/> | ||
=='''Introduction'''== | =='''Introduction'''== | ||
| - | |||
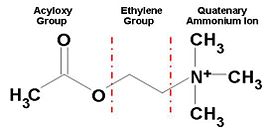
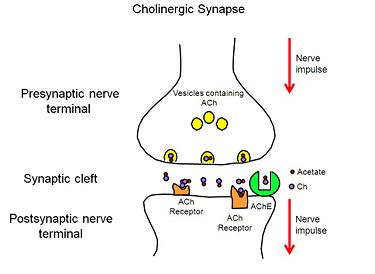
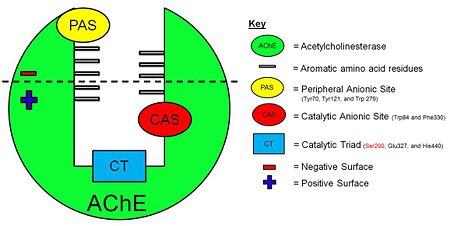
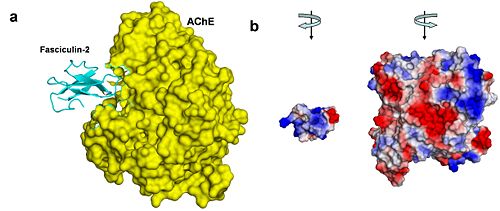
[[Acetylcholinesterase]] (AChE) is essential for hydrolysis of the neurotransmitter acetylcholine (ACh), and, therefore, for termination of impulse transmission at cholinergic synapses (Figure 2). Irreversible inhibition of AChE can result in accumulation of ACh at cholinergic synapses and, ultimately, to death. Conversely, decreased levels of ACh may result in the memory deficits associated with Alzheimer's disease<ref>PMID: 14501022</ref>. AChE has a deep (20Å) and narrow (5Å) gorge lined with 14 aromatic residues, with its active site located near the bottom of the gorge<ref>PMID: 1678899</ref>. Initially, ACh binds to the peripheral anionic site (PAS) of AChE, and is funneled down the gorge to the active site by interactions between its quaternary ammonium group and the aromatic rings of 14 aromatic amino acid residues lining the gorge. At the active site, ACh is oriented for hydrolysis by interactions between the catalytic anionic site and its quaternary ammonium group. Fasciculin-II (FAS-II), a potent polypeptide toxin present in the venom of the East African green mamba (Dendroaspis angusticeps), inhibits AChE by binding to the top of the active-site gorge, interacting tightly with residues that form the PAS; it thus prevents ACh from entering the active-site gorge<ref>PMID:8747462</ref>. The Hostos-Lincoln Academy Students Modeling A Research Topic (S.M.A.R.T) team and the Center for BioMolecular Modeling have designed and fabricated two physical models using a combination of computational molecular modeling and three-dimensional (3D) printing technology: ''Torpedo californica'' (''Tc'') AChE complexed with a modeled ACh molecule ligand, and a complex of FAS-II with ''Tc''AChE. | [[Acetylcholinesterase]] (AChE) is essential for hydrolysis of the neurotransmitter acetylcholine (ACh), and, therefore, for termination of impulse transmission at cholinergic synapses (Figure 2). Irreversible inhibition of AChE can result in accumulation of ACh at cholinergic synapses and, ultimately, to death. Conversely, decreased levels of ACh may result in the memory deficits associated with Alzheimer's disease<ref>PMID: 14501022</ref>. AChE has a deep (20Å) and narrow (5Å) gorge lined with 14 aromatic residues, with its active site located near the bottom of the gorge<ref>PMID: 1678899</ref>. Initially, ACh binds to the peripheral anionic site (PAS) of AChE, and is funneled down the gorge to the active site by interactions between its quaternary ammonium group and the aromatic rings of 14 aromatic amino acid residues lining the gorge. At the active site, ACh is oriented for hydrolysis by interactions between the catalytic anionic site and its quaternary ammonium group. Fasciculin-II (FAS-II), a potent polypeptide toxin present in the venom of the East African green mamba (Dendroaspis angusticeps), inhibits AChE by binding to the top of the active-site gorge, interacting tightly with residues that form the PAS; it thus prevents ACh from entering the active-site gorge<ref>PMID:8747462</ref>. The Hostos-Lincoln Academy Students Modeling A Research Topic (S.M.A.R.T) team and the Center for BioMolecular Modeling have designed and fabricated two physical models using a combination of computational molecular modeling and three-dimensional (3D) printing technology: ''Torpedo californica'' (''Tc'') AChE complexed with a modeled ACh molecule ligand, and a complex of FAS-II with ''Tc''AChE. | ||
| Line 69: | Line 70: | ||
{{clear}} | {{clear}} | ||
| - | + | __TOC__ | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
=='''References'''== | =='''References'''== | ||
<references/> | <references/> | ||
| Line 80: | Line 76: | ||
=='''Acknowledgements'''== | =='''Acknowledgements'''== | ||
| - | |||
1. Howard Hughes Medical Institue Pre-College Program | 1. Howard Hughes Medical Institue Pre-College Program | ||
| Line 98: | Line 93: | ||
9. Malcolm Twist | 9. Malcolm Twist | ||
| - | + | ||
| - | + | __NOTOC__ | |
Current revision
| |||||||||||
Acknowledgements
1. Howard Hughes Medical Institue Pre-College Program
2. Center for BioMolecular Modeling, Milwaukee School of Engineering
3. The Rockefeller University Center for Clinical and Translational Science
4. The Rockefeller University S.M.A.R.T Team Program
5. The Rockefeller University Science Outreach Program
6. Touro College of Pharmacy
7. Michal Harel, Weizmann Institute of Science
8. Natural Sciences Department,Hostos Community College, Bronx, NY
9. Malcolm Twist
Proteopedia Page Contributors and Editors (what is this?)
Joel L. Sussman, Alexander Berchansky, Jaime Prilusky, Michal Harel, Daviana Dueno, Randol Mata, Mary Acheampong, Allison Granberry, Alafia Henry, Marisa L. VanBrakle
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.