User:Michael O'Shaughnessy/ TS
From Proteopedia
| (19 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
== Overview == | == Overview == | ||
| - | The enzyme Thymidylate Synthase (TS) catalyzes the transfer of a methyl group and a hydride from 5,10-methylenetetrahydrofolate to 2-deoxyuridine-5'-monophosphate, resulting in the formation of thymidine 5'-monophosphate and dihydrofolate. This is the only de novo source of dTMP (a precursor to Thymine) in humans. <ref>DOI 10.1021/acs.biochem.1c00063</ref> | + | This page is a work in progress during the spring 2022 semester. |
| + | |||
| + | |||
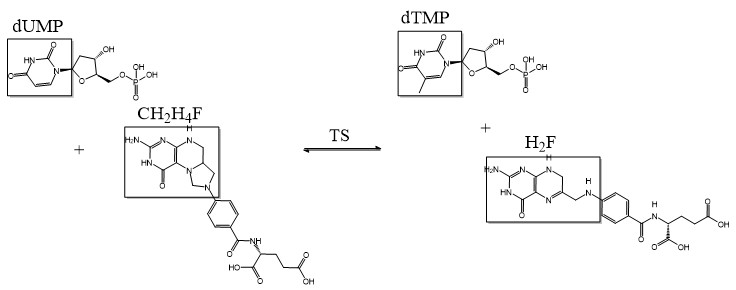
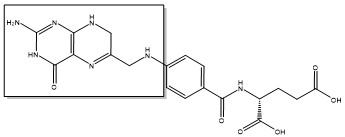
| + | The enzyme Thymidylate Synthase (TS) is an important enzyme in one-carbon metabolism[https://proteopedia.org/wiki/index.php/One-carbon_metabolism]. TS catalyzes the transfer of a methyl group and a hydride from 5,10-methylenetetrahydrofolate to 2-deoxyuridine-5'-monophosphate, resulting in the formation of thymidine 5'-monophosphate and dihydrofolate. This is the only de novo source of dTMP (a precursor to Thymine) in humans. <ref>DOI 10.1021/acs.biochem.1c00063</ref> | ||
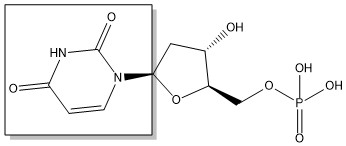
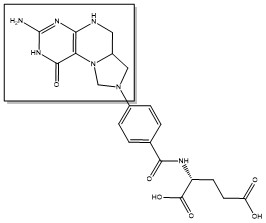
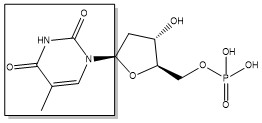
2-deoxyuridine-5'-monophosphate(dUMP) + 5, 10-methylenetetrahydrofolate(CH<sub>2</sub>H<sub>4</sub>F) ⇌ thymidine 5'-monophosphate(dTMP) + Dihydrofolate(H<sub>2</sub>F) | 2-deoxyuridine-5'-monophosphate(dUMP) + 5, 10-methylenetetrahydrofolate(CH<sub>2</sub>H<sub>4</sub>F) ⇌ thymidine 5'-monophosphate(dTMP) + Dihydrofolate(H<sub>2</sub>F) | ||
| + | |||
| + | [[Image:TS Overview.jpg]] | ||
== Disease == | == Disease == | ||
== Relevance == | == Relevance == | ||
| - | Due to its role in cell division, thymidylate synthase has become a popular target for anticancer drugs. Indirect inhibition of thymidylate synthase by the drug 5-fluorouracil (5-FU) is one of the most used inhibitors for study of TS function. This drug indirectly inhibits TS as it it eventually converted to FdUMP, which forms a covalent complex with both the active site cysteine and CH<sub>2</sub>H<sub>4</sub>F. Inhibition of TS | + | Due to its role in cell division, thymidylate synthase has become a popular target for anticancer drugs. Indirect inhibition of thymidylate synthase by the drug 5-fluorouracil (5-FU) is one of the most used inhibitors for study of TS function. This drug indirectly inhibits TS as it it eventually converted to FdUMP, which forms a covalent complex with both the active site cysteine and CH<sub>2</sub>H<sub>4</sub>F. Inhibition of TS halts the production of dTMP and, indirectly, 2'-deoxythymidine-5'-triphosphate (dTTP). Both dTMP and dTTP are essential building blocks for DNA synthesis and their absence halts the ability of cells to replicate their genetic information. This is especially effective in cancer cells that rapidly divide and require large amounts of dTMP and dTTP. <ref>DOI 10.2174/0929867054864868</ref> |
<StructureSection load= '7jxf' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load= '7jxf' size='340' side='right' caption='Caption for this structure' scene=''> | ||
| - | + | ||
| - | + | ||
<scene name='90/907470/Ts/1'>Text To Be Displayed</scene> | <scene name='90/907470/Ts/1'>Text To Be Displayed</scene> | ||
| Line 18: | Line 23: | ||
<scene name='90/907470/Ts/2'>TS with no DDT or UMP</scene> | <scene name='90/907470/Ts/2'>TS with no DDT or UMP</scene> | ||
| - | <ref> | + | <ref>PMID:11590022</ref> |
| Line 40: | Line 45: | ||
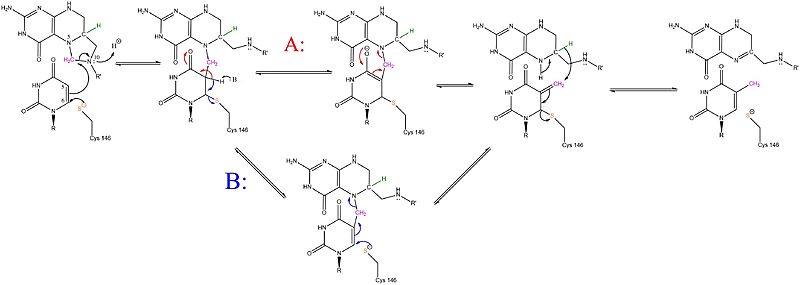
==Mechanism== | ==Mechanism== | ||
| - | [[Image:TS Mechanism.jpg]] | + | [[Image:TS Mechanism.jpg|800px]] |
== Structural highlights == | == Structural highlights == | ||
| - | The active site Cysteine has two <scene name='90/907470/Ts_7jxf_percentb/ | + | The active site Cysteine has two <scene name='90/907470/Ts_7jxf_percentb/3'>conformations</scene>. |
<jmol> | <jmol> | ||
<jmolRadioGroup> | <jmolRadioGroup> | ||
| Line 64: | Line 69: | ||
</item> | </item> | ||
</jmolRadioGroup> | </jmolRadioGroup> | ||
| - | </ | + | <jmolLink> |
| + | <script> | ||
| + | define current selected; | ||
| + | select [VNM]301:B.NA4%B #9178; | ||
| + | selectionHalos on; | ||
| + | delay 0.5; | ||
| + | selectionHalos off; | ||
| + | select current; | ||
| + | </script> | ||
| + | <text>☼</text> | ||
| + | </jmolLink> | ||
| + | </jmol> | ||
Current revision
Contents |
Overview
This page is a work in progress during the spring 2022 semester.
The enzyme Thymidylate Synthase (TS) is an important enzyme in one-carbon metabolism[1]. TS catalyzes the transfer of a methyl group and a hydride from 5,10-methylenetetrahydrofolate to 2-deoxyuridine-5'-monophosphate, resulting in the formation of thymidine 5'-monophosphate and dihydrofolate. This is the only de novo source of dTMP (a precursor to Thymine) in humans. [1]
2-deoxyuridine-5'-monophosphate(dUMP) + 5, 10-methylenetetrahydrofolate(CH2H4F) ⇌ thymidine 5'-monophosphate(dTMP) + Dihydrofolate(H2F)
Disease
Relevance
Due to its role in cell division, thymidylate synthase has become a popular target for anticancer drugs. Indirect inhibition of thymidylate synthase by the drug 5-fluorouracil (5-FU) is one of the most used inhibitors for study of TS function. This drug indirectly inhibits TS as it it eventually converted to FdUMP, which forms a covalent complex with both the active site cysteine and CH2H4F. Inhibition of TS halts the production of dTMP and, indirectly, 2'-deoxythymidine-5'-triphosphate (dTTP). Both dTMP and dTTP are essential building blocks for DNA synthesis and their absence halts the ability of cells to replicate their genetic information. This is especially effective in cancer cells that rapidly divide and require large amounts of dTMP and dTTP. [2]
| |||||||||||
References
- ↑ Kholodar SA, Finer-Moore JS, Swiderek K, Arafet K, Moliner V, Stroud RM, Kohen A. Caught in Action: X-ray Structure of Thymidylate Synthase with Noncovalent Intermediate Analog. Biochemistry. 2021 Apr 8. doi: 10.1021/acs.biochem.1c00063. PMID:33829766 doi:http://dx.doi.org/10.1021/acs.biochem.1c00063
- ↑ Costi MP, Ferrari S, Venturelli A, Calo S, Tondi D, Barlocco D. Thymidylate synthase structure, function and implication in drug discovery. Curr Med Chem. 2005;12(19):2241-58. doi: 10.2174/0929867054864868. PMID:16178783 doi:http://dx.doi.org/10.2174/0929867054864868
- ↑ Fritz TA, Tondi D, Finer-Moore JS, Costi MP, Stroud RM. Predicting and harnessing protein flexibility in the design of species-specific inhibitors of thymidylate synthase. Chem Biol. 2001 Oct;8(10):981-95. PMID:11590022