Sandbox Reserved 1717
From Proteopedia
(Difference between revisions)
| (11 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
<StructureSection load='' size='350' side='right' caption='Structure of Closed Vitamin K Epoxide Reductase (PDB entry [[6wv3]])' scene='90/904321/Closedconformation/2'> | <StructureSection load='' size='350' side='right' caption='Structure of Closed Vitamin K Epoxide Reductase (PDB entry [[6wv3]])' scene='90/904321/Closedconformation/2'> | ||
| - | |||
| - | |||
== Introduction == | == Introduction == | ||
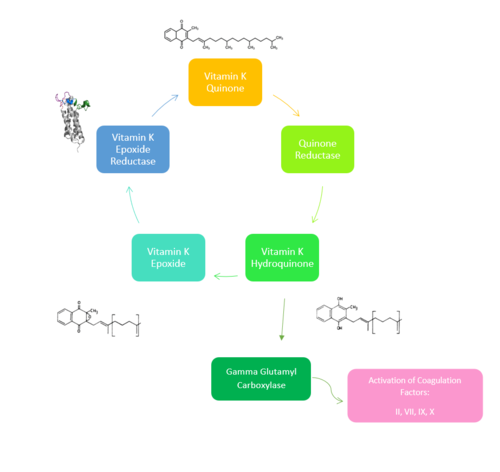
| - | [[Image:NewVitaminKCycle.PNG| | + | [[Image:NewVitaminKCycle.PNG|500px|right|thumb|'''Figure 1. Overview of Vitamin K Cycle''': The cycle begins with [https://en.wikipedia.org/wiki/Vitamin_K Vitamin K Quinone]. Vitamin K Quinone is reduced by enzyme Quinone Reductase. This leaves Vitamin K Hydroquinone which can either lead to [https://en.wikipedia.org/wiki/Gamma-glutamyl_carboxylase Gamma Carboxylase]activity that will activate Blood Coagulation Factors II, VII, IX, and X. After this, Vitamin K Epoxide is left over. Vitamin K Epoxide is reduced by the enzyme Vitamin K Epoxide Reductase to reform Vitamin K Quinone. ]] |
<scene name='90/904321/Vitamin_k_epoxide_reductase/1'>Vitamin K Epoxide Reductase</scene> | <scene name='90/904321/Vitamin_k_epoxide_reductase/1'>Vitamin K Epoxide Reductase</scene> | ||
| - | [https://en.wikipedia.org/wiki/Vitamin_K_epoxide_reductase | + | [https://en.wikipedia.org/wiki/Vitamin_K_epoxide_reductase (VKOR)] is an endoplasmic membrane enzyme that generates the active form of Vitamin K to support blood coagulation. VKOR homologs are integral membrane thiol oxidoreductases [https://en.wikipedia.org/wiki/Thiol_oxidoreductase Thiol OxidoReductase] due to the function of VKOR being dependent on thiol residues and disulfide bonding. The Vitamin K Cycle and the VKOR enzyme specifically are common drug targets for thromboembolic diseases. This is because, as pictured, the vitamin K cycle is required to activate blood coagulant factors [https://en.wikipedia.org/wiki/Thrombin II], [https://en.wikipedia.org/wiki/Coagulation_factor_VII VII], [https://en.wikipedia.org/wiki/Factor_IX IX], and [https://en.wikipedia.org/wiki/Factor_X#:~:text=Factor%20X%2C%20also%20known%20by,vitamin%20K%20for%20its%20synthesis. X]. Coagulant factor activation promotes blood clotting, which in high amounts can be dangerous and cause thromboembolic diseases such as stroke, deep vein thrombosis, and/or pulmonary embolism. Vitamin K Epoxide Reductase is found and primarily synthesized in the liver. It is embedded in the membrane known as the endoplasmic reticulum. |
===Reaction=== | ===Reaction=== | ||
| Line 27: | Line 25: | ||
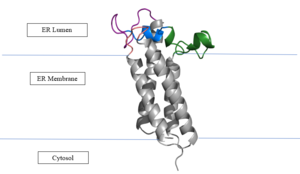
| - | [[Image:VKORmembrane.png|300px|left|thumb|Figure | + | [[Image:VKORmembrane.png|300px|left|thumb|Figure 2. Orientation in Endoplasmic Reticulum: The cap region is partially oriented in the ER Lumen, however the active site remains within the ER membrane. The Beta Hairpin, Loop 3-4, Cap Loop are all in the ER Lumen. The Anchor is partially within the ER lumen, and partially embedded in the ER membrane. The anchor is what attaches the cap domain and stabilizes it, which allows the cap domain to cover the active site. ]] |
The reaction catalyzed by VKOR is a redox reaction. ''Vitamin K Epoxide => Vitamin K Quinone'' Vitamin K Epoxide is reduced by transferring two electrons through a disulfide bond. These disulfide bonds come from the conserved cysteines. This redox reaction that is catalyzed by VKOR produces Vitamin K Quinone. | The reaction catalyzed by VKOR is a redox reaction. ''Vitamin K Epoxide => Vitamin K Quinone'' Vitamin K Epoxide is reduced by transferring two electrons through a disulfide bond. These disulfide bonds come from the conserved cysteines. This redox reaction that is catalyzed by VKOR produces Vitamin K Quinone. | ||
| Line 47: | Line 45: | ||
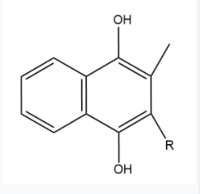
== Vitamin K Epoxide == | == Vitamin K Epoxide == | ||
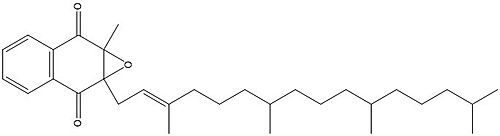
| - | [[Image:Vitamin K epoxide.jpg|500 px|right|thumb|Figure | + | [[Image:Vitamin K epoxide.jpg|500 px|right|thumb|Figure 3. Vitamin K Epoxide structure]] |
As mentioned above, Vitamin K epoxide is a part of the Vitamin K cycle and required for blood coagulation. In the cycle, VKOR reduces Vitamin K epoxide to Vitamin K Quinone, or the active form of Vitamin K. In this conversion, VKOR donates electrons to Vitamin K epoxide from the S-H of the active pair of cysteines, C132-C135. The mediated cysteine pair, C43-C51, has to be reduced for the transfer of electrons to the substrate to occur. | As mentioned above, Vitamin K epoxide is a part of the Vitamin K cycle and required for blood coagulation. In the cycle, VKOR reduces Vitamin K epoxide to Vitamin K Quinone, or the active form of Vitamin K. In this conversion, VKOR donates electrons to Vitamin K epoxide from the S-H of the active pair of cysteines, C132-C135. The mediated cysteine pair, C43-C51, has to be reduced for the transfer of electrons to the substrate to occur. | ||
| - | Two other notable structures are Vitamin K Quinone (Fig. 5) and Vitamin K Hydroquinone (Fig. 6). Vitamin K Quinone is the product that is released after the reaction with Vitamin K Epoxide and VKOR. (Fig. | + | Two other notable structures are Vitamin K Quinone (Fig. 5) and Vitamin K Hydroquinone (Fig. 6). Vitamin K Quinone is the product that is released after the reaction with Vitamin K Epoxide and VKOR. (Fig. 1) |
| - | [[Image:Vitaminkquinone.PNG|200 px|left|thumb|Figure | + | [[Image:Vitaminkquinone.PNG|200 px|left|thumb|Figure 4. Vitamin K Quinone structure]] [[Image:Vitaminkhydroquinone.PNG|200 px|right|thumb|Figure 5. Vitamin K Hydroquinone structure]] |
=== Binding === | === Binding === | ||
| - | In its resting state, VKOR is in its <scene name='90/904322/Open_conformation/ | + | In its resting state, VKOR is in its <scene name='90/904322/Open_conformation/2'>open conformation</scene>. The Vitamin K epoxide enters through the <scene name='90/904322/Tunnel/7'>isoprenyl-chain tunnel</scene>. The tunnel is located between <scene name='90/904322/Tunnel/8'>TM2 and TM3</scene>.<ref name="Li">PMID:20110994</ref> The carbonyls on the VK epoxide bind to <scene name='90/904322/Vko_binding/2'>Asn80 and Tyr139</scene> on VKOR. With Vitamin K epoxide bound, the conformation transitions from open to closed, where the catalytic process will begin. |
| - | + | ||
| - | + | ||
| - | + | ||
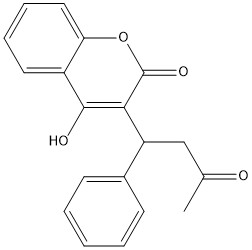
== Warfarin == | == Warfarin == | ||
[https://en.wikipedia.org/wiki/Warfarin Warfarin] is the most common [https://en.wikipedia.org/wiki/Vitamin_K_antagonist Vitamin K antagonist (VKA)]. Warfarin is a competitive inhibitor, taking the place of Vitamin K Epoxide (VKO) in the active site of Vitamin K Epoxide Reductase (VKOR). When warfarin binds in the active site, it causes VKOR to go into the closed conformation. | [https://en.wikipedia.org/wiki/Warfarin Warfarin] is the most common [https://en.wikipedia.org/wiki/Vitamin_K_antagonist Vitamin K antagonist (VKA)]. Warfarin is a competitive inhibitor, taking the place of Vitamin K Epoxide (VKO) in the active site of Vitamin K Epoxide Reductase (VKOR). When warfarin binds in the active site, it causes VKOR to go into the closed conformation. | ||
| - | [[Image:warfarin.jpg|400 px| | + | [[Image:warfarin.jpg|400 px|left|thumb|Figure 6. Warfarin structure]] |
=== Binding === | === Binding === | ||
| - | Warfarin still forms Hydrogen bonds with <scene name='90/904322/ | + | Warfarin still forms Hydrogen bonds with <scene name='90/904322/Asn80_tyr139_warfarin/1'>Asn80 and Tyr139</scene>. The specific bonds are between Asn80 and the 2-ketone group of warfarin and Tyr139 with the 4-hydroxyl group of warfarin. The rest of the pocket is hydrophobic interactions. The H bonds are necessary for the recognition of the ligand in the binding site of VKOR. |
| + | |||
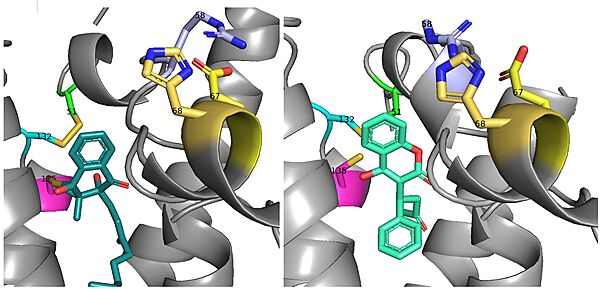
| + | There is a slight difference in the way in which warfarin binds compared to VKO. Warfarin binds are a slightly different angle (Fig.7). This creates a difference in how the cap loop and anchor domain interact, and that noticeable difference is with <scene name='90/904322/Arg58/4'>Arg58</scene>. With VKO, Arg58, located in the cap loop, directly interacts with <scene name='90/904322/Arg58_vko/5'>Glu67</scene> when VKO is bound. When warfarin binds, Arg58 is found inserted between <scene name='90/904322/Arg58_warfarin/2'>Glu67 and His68</scene> of the anchor domain.<ref name=”Liu”>PMID:33154105</ref> | ||
| + | [[Image:VKO and Warfarin binding.jpg|600 px|right|thumb|Figure 6. The slight angle change in which VKO(left) and warfarin(right) bind. The location of the cap domain and how it differs between each is apparent.]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| - | There is a slight difference in the way in which warfarin binds compared to VKO. Warfarin binds are a slightly different angle. This creates a difference in how the cap loop and anchor domain interact, and that noticeable difference is with <scene name='90/904322/Arg58/4'>Arg58</scene>. With VKO, Arg58, located in the cap loop, directly interacts with <scene name='90/904322/Arg58_vko/5'>Glu67</scene> when VKO is bound. When warfarin binds, Arg58 is found inserted between<scene name='90/904322/Arg58_warfarin/2'>Glu67 and His68</scene> of the anchor domain. | ||
=== Disease === | === Disease === | ||
[https://en.wikipedia.org/wiki/Vitamin_K_antagonist#:~:text=Vitamin%20K%20antagonists%20(VKA)%20are,the%20recycling%20of%20vitamin%20K. Vitamin K Antagonists] play a big role in the treatment of thromboembolic diseases, like a stroke or heart attack.<ref name="Goy">PMID:23034830</ref> [https://en.wikipedia.org/wiki/Warfarin Warfarin] is the most common medication for this treatment, acting as a blood thinner. Warfarin binding in VKOR overall prevents the triggering of coagulation factors that form blood clots. | [https://en.wikipedia.org/wiki/Vitamin_K_antagonist#:~:text=Vitamin%20K%20antagonists%20(VKA)%20are,the%20recycling%20of%20vitamin%20K. Vitamin K Antagonists] play a big role in the treatment of thromboembolic diseases, like a stroke or heart attack.<ref name="Goy">PMID:23034830</ref> [https://en.wikipedia.org/wiki/Warfarin Warfarin] is the most common medication for this treatment, acting as a blood thinner. Warfarin binding in VKOR overall prevents the triggering of coagulation factors that form blood clots. | ||
| + | |||
| + | |||
| + | |||
| + | |||
==References== | ==References== | ||
<references/> | <references/> | ||
Current revision
| |||||||||||