We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
VEGF signaling pathway
From Proteopedia
(Difference between revisions)
| (5 intermediate revisions not shown.) | |||
| Line 3: | Line 3: | ||
[[Vascular Endothelial Growth Factor]]s (VEGFs) are a class of proteins that regulate vascular development in embryos and angiogenesis in adult mammals after sustaining an injury or notably in [[Cancer|cancerous]] tumors. A number of structural studies have been conducted on VEGF and its receptors (VEGFRs) to better understand the VEGF-VEGFR interaction and how the signal cascade originating from this interaction leads to a number of biological features. VEGF and its receptors have been closely looked at for their potential use as targets for pharmaceutical medicine with some success. The VEGF family contains VEGF-A which mediates increased vascular permeability, VEGF-B which is a growth factor, VEGF-C is active in angiogenesis, VEGF-E is found in viruses and VEGF-F in snake venom.<br /> For additional information see:<br /> | [[Vascular Endothelial Growth Factor]]s (VEGFs) are a class of proteins that regulate vascular development in embryos and angiogenesis in adult mammals after sustaining an injury or notably in [[Cancer|cancerous]] tumors. A number of structural studies have been conducted on VEGF and its receptors (VEGFRs) to better understand the VEGF-VEGFR interaction and how the signal cascade originating from this interaction leads to a number of biological features. VEGF and its receptors have been closely looked at for their potential use as targets for pharmaceutical medicine with some success. The VEGF family contains VEGF-A which mediates increased vascular permeability, VEGF-B which is a growth factor, VEGF-C is active in angiogenesis, VEGF-E is found in viruses and VEGF-F in snake venom.<br /> For additional information see:<br /> | ||
[[VEGFR]]<br /> | [[VEGFR]]<br /> | ||
| - | [[VEGF IN COMPLEX WITH A NEUTRALIZING ANTIBODY]] | + | [[VEGF IN COMPLEX WITH A NEUTRALIZING ANTIBODY]] |
| + | |||
<br /> | <br /> | ||
<br /> | <br /> | ||
[[Image: VEGF_effects.PNG|350px|left|thumb| Interaction of VEGFs with VEGFRs. ]] | [[Image: VEGF_effects.PNG|350px|left|thumb| Interaction of VEGFs with VEGFRs. ]] | ||
{{Clear}} | {{Clear}} | ||
| - | ==History and Biological Function== | + | ===History and Biological Function=== |
VEGF-A was first described by Senger ''et al.'' in 1983 as a tumor secreted “vascular-permeability factor (VPF). <ref>PMID:6823562</ref> In 1989, Henzel and Ferrara reported the isolation of an endothelial cell mitogen they named VEGF which also mediated vascular permeability in vivo. Subsequent sequencing revealed that VPF and VEGF were identical, with the VEGF moniker sticking. <ref>PMID: 2735925</ref>. VEGF represents a family of homodimeric glycoprotins which are essential for vasculogenesis (embryonic development of blood vessels), Lymphangiogenesis (lymphatic system development) and angiogenesis (formation of new blood vessels from pre-existing ones). <ref name="Ferrara">PMID:15294883</ref> VEGF-A, arguably the most important member of the VEGF family, belongs to a gene family that includes placenta growth factor (PIGF) and VEGF’s B, C, D, E (Viral), and F (found in snake toxin) <ref>PMID:15542594</ref>. | VEGF-A was first described by Senger ''et al.'' in 1983 as a tumor secreted “vascular-permeability factor (VPF). <ref>PMID:6823562</ref> In 1989, Henzel and Ferrara reported the isolation of an endothelial cell mitogen they named VEGF which also mediated vascular permeability in vivo. Subsequent sequencing revealed that VPF and VEGF were identical, with the VEGF moniker sticking. <ref>PMID: 2735925</ref>. VEGF represents a family of homodimeric glycoprotins which are essential for vasculogenesis (embryonic development of blood vessels), Lymphangiogenesis (lymphatic system development) and angiogenesis (formation of new blood vessels from pre-existing ones). <ref name="Ferrara">PMID:15294883</ref> VEGF-A, arguably the most important member of the VEGF family, belongs to a gene family that includes placenta growth factor (PIGF) and VEGF’s B, C, D, E (Viral), and F (found in snake toxin) <ref>PMID:15542594</ref>. | ||
| Line 40: | Line 41: | ||
[[VEGF 3D Structures]] | [[VEGF 3D Structures]] | ||
| + | ==[[Vascular Endothelial Growth Factor Receptor]]== | ||
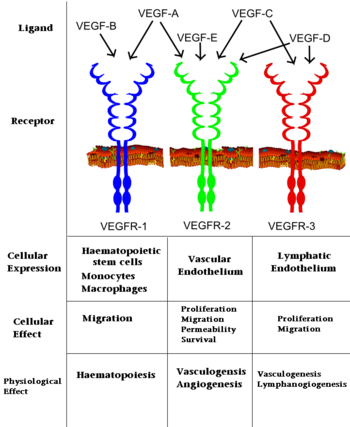
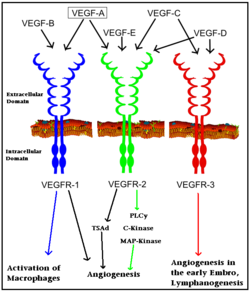
| + | [[Vascular Endothelial Growth Factor Receptor]]s (VEGFRs) are [[tyrosine kinase receptors]] responsible for binding with [[VEGF]] to initiate signal cascades that stimulate angiogenesis among other effects<ref>PMID:22130231</ref>. VEGFRs convey signals to other signal transduction effectors via autophosphorylation of specific residues in its structure. Because VEGFRs are up-regulated in cancerous tumors which have a high metabolic need for oxygen, VEGFRs are an important target for [[pharmaceutical drugs]] treating [[cancer]]. VEGFR subtypes are numbered 1,2,3. | ||
| + | [[Image: VEGF_receptors.png|250px|left|thumb| Interaction of VEGFs with VEGFRs. Colored arrows indicate major pathway. Black arrows indicate minor pathway.]] | ||
| + | {{Clear}} | ||
| + | |||
| + | See also [[Kinase-linked, enzyme-linked and related receptors]]. | ||
| + | |||
| + | ===Biological Function === | ||
| + | |||
| + | The VEGFRs are a family of tyrosine kinase receptors on the surface of different cells depending on family identity. VEGFR-1 is expressed on haematopoietic stem cells, monocytes, and vascular endothelial cells. VEGFR-2 is expressed on vascular endothelial cells and lymphatic endothelial cells, while VEGFR-3 is only expressed on lymphatic endothelial cells<ref>PMID:16633338</ref>. | ||
| + | |||
| + | In terms of function, VEGFR-1 is required for the recruitment of haematopoietic stem cells as well as the migration of monocytes and macrophages while VEGFR-2 regulates vascular endothelial function and VEGFR-3 regulates lymphatic endothelial cell function.<ref>PMID: 17658244</ref> VEGFR-2 has been the focus of the most research as it is the major signal transducer of both physioligcal, and perhaps more importantly, pathological angiogenesis, especially in cancerous tumors. VEGFR-2 is of critical importance to the body as exemplified by Shalaby ''et al.'' who demonstrated that VEGFR-2 gene knockout mice die at E8-8.5 due to lack of vasculogenesis.<ref>PMID:7596453</ref> The signal cascade initiated by binding VEGF to VEGFR is dependent upon specific sites of phosphorylation in the VEGFR structure and the interaction between these phosphorylated sites and other signaling molecules. | ||
| + | |||
| + | ===Structure of VEGFR-2 and Biology=== | ||
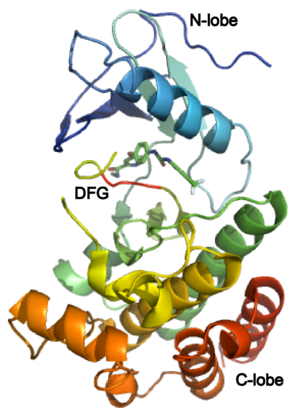
| + | The structure of VEGFR-2 can been seen at the right. VEGF-A binds to the second and third extracellular Ig-like domains of VEGFR-2 with a 10-fold lower affinity than it does to the second Ig-like domain of VEGFR-1, despite the fact that VEGFR-2 is the principal mediator of several physiological effects on endothelial cells including proliferation, migration, and survival.<ref> PMID:9813036</ref> Binding of VEGF to the domains 2 and 3 of a VEGFR-2 monomer increases the probability that an additional VEGFR-2 binds the tethered ligand to form a dimmer. Once the two receptors are cross-linked, interactions between their membrane-proximal domain 7s stabilize the dimmer significantly. This dimerization and stabilization allows for precise positioning of the intracellular kinase domains, resulting in autophosphorylation and subsequent activation of the classical extracellular signal-regulated kinases (ERK) pathway.<ref>PMID:17293873</ref>. | ||
| + | |||
| + | The tyrosine kinase domain of VEGFR-2 is separated into two segments with a 70 amino acid long kinase insert region. Upon binding VEGFA and subsequent dimerization, VEGFR-2 is autophosphoryalted at the carboxy terminal tail and kinase insert region. Six tyrosine residues of VEGFR2 are autophosphorylated (see Fig.1<ref>PMID:15962004</ref>). <scene name='41/411436/Cv/2'>Auto-phosphorylation of residues1054 and 1059</scene> within the activation loop of VEGFR2 leads to increased kinase activity<ref>PMID:10037737</ref>.<br /> | ||
| + | |||
| + | ===Medical significance=== | ||
| + | [[Image: Sorafenib.png|300px|left|thumb| [[Sorafenib]], anti VEGFR drug targeting the MAP Kinase pathway, marketed by Bayer for Renal and Liver [[Cancer]].]] | ||
| + | {{Clear}} | ||
| + | VEGFRs play a critical role in a number of signal transduction pathways essential for angiogenesis and cell migration. VEGFR is a particularly attractive target because they are expressed almost exclusively in endothelial cells and are highly upregulated in many tumor endothelium types.<ref>PMID:12360282</ref> In fact, work by Plate ''et al.'' revealed that VEGFR-2 expression is 5 fold higher in the tumor vasculature than in normal vasculature. This increased VEGFR-2 expression is due to a cancer cells high metabolic demand for oxygen and other nutrients to continue growing, thus requiring a vast vasculature. VEGFR signaling has also been implicated in diabetic reinopathy and the progression of rheumatoid arthritis and atherosclerosis.<ref>PMID:17826917</ref> | ||
| + | |||
| + | Bevacizumab ([[Avastin]]) is a recombinant [[monoclonal antibody]] marketed by the pharmaceutical company Roche. It earned over $5 billion dollars in 2009 treating a number of cancers. It’s principal mechanism of action is as an anti-VEGF antibody that favors antiangiogenesis in the tumor microenvironment while effecting the rest of the body to a lesser extent. It has been found to decrease tumor vascular permeability subsequently reducing the delivery of oxygen and nutrients to cancer cells when used in combination with chemotherapy.<ref>PMID:11533692 </ref> Other drugs target VEGFR such as [[Sorafenib]] ([[Nexavar]]), [[Sunitinib]] ([[Sutent]]) and Vandetanib, binding to various parts of the receptor, either preventing interaction with [[VEGF]] or with other downstream signaling molecules. | ||
| + | |||
| + | *<scene name='41/411436/Cv/4'>Anti-tumor inhibitor binding site</scene> (PDB code [[3c7q]]). | ||
| + | <br /> | ||
| + | ===[[3D structures of vascular endothelial growth factor receptor]]=== | ||
==Additional Resources== | ==Additional Resources== | ||
For additional information, see: | For additional information, see: | ||
Current revision
| |||||||||||
References
- ↑ Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983 Feb 25;219(4587):983-5. PMID:6823562
- ↑ Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jun 15;161(2):851-8. PMID:2735925
- ↑ Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004 Aug;25(4):581-611. PMID:15294883 doi:10.1210/er.2003-0027
- ↑ Suto K, Yamazaki Y, Morita T, Mizuno H. Crystal structures of novel vascular endothelial growth factors (VEGF) from snake venoms: insight into selective VEGF binding to kinase insert domain-containing receptor but not to fms-like tyrosine kinase-1. J Biol Chem. 2005 Jan 21;280(3):2126-31. Epub 2004 Nov 12. PMID:15542594 doi:10.1074/jbc.M411395200
- ↑ Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992 Oct 29;359(6398):843-5. PMID:1279431 doi:http://dx.doi.org/10.1038/359843a0
- ↑ Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997 Feb;18(1):4-25. PMID:9034784
- ↑ Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996 Apr 4;380(6573):435-9. PMID:8602241 doi:http://dx.doi.org/10.1038/380435a0
- ↑ Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996 Apr 4;380(6573):435-9. PMID:8602241 doi:http://dx.doi.org/10.1038/380435a0
- ↑ Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001 Mar;114(Pt 5):853-65. PMID:11181169
- ↑ Muller YA, Li B, Christinger HW, Wells JA, Cunningham BC, de Vos AM. Vascular endothelial growth factor: crystal structure and functional mapping of the kinase domain receptor binding site. Proc Natl Acad Sci U S A. 1997 Jul 8;94(14):7192-7. PMID:9207067
- ↑ Keyt BA, Nguyen HV, Berleau LT, Duarte CM, Park J, Chen H, Ferrara N. Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors. Generation of receptor-selective VEGF variants by site-directed mutagenesis. J Biol Chem. 1996 Mar 8;271(10):5638-46. PMID:8621427
- ↑ Pieren M, Prota AE, Ruch C, Kostrewa D, Wagner A, Biedermann K, Winkler FK, Ballmer-Hofer K. Crystal structure of the Orf virus NZ2 variant of vascular endothelial growth factor-E. Implications for receptor specificity. J Biol Chem. 2006 Jul 14;281(28):19578-87. Epub 2006 May 3. PMID:16672228 doi:10.1074/jbc.M601842200
- ↑ Oefner C, D'Arcy A, Winkler FK, Eggimann B, Hosang M. Crystal structure of human platelet-derived growth factor BB. EMBO J. 1992 Nov;11(11):3921-6. PMID:1396586
- ↑ Pieren M, Prota AE, Ruch C, Kostrewa D, Wagner A, Biedermann K, Winkler FK, Ballmer-Hofer K. Crystal structure of the Orf virus NZ2 variant of vascular endothelial growth factor-E. Implications for receptor specificity. J Biol Chem. 2006 Jul 14;281(28):19578-87. Epub 2006 May 3. PMID:16672228 doi:10.1074/jbc.M601842200
- ↑ Errico M, Riccioni T, Iyer S, Pisano C, Acharya KR, Persico MG, De Falco S. Identification of placenta growth factor determinants for binding and activation of Flt-1 receptor. J Biol Chem. 2004 Oct 15;279(42):43929-39. Epub 2004 Jul 21. PMID:15272021 doi:10.1074/jbc.M401418200
- ↑ Brockington A, Lewis C, Wharton S, Shaw PJ. Vascular endothelial growth factor and the nervous system. Neuropathol Appl Neurobiol. 2004 Oct;30(5):427-46. PMID:15488020 doi:10.1111/j.1365-2990.2004.00600.x
- ↑ Doyle B, Morton JP, Delaney DW, Ridgway RA, Wilkins JA, Sansom OJ. p53 mutation and loss have different effects on tumourigenesis in a novel mouse model of pleomorphic rhabdomyosarcoma. J Pathol. 2010 Jun 17. PMID:20662002 doi:10.1002/path.2748
- ↑ http://www.foxbusiness.com/story/markets/industries/technology/roche-increased-avastin-sales-efforts-doubles-force/
- ↑ Takahashi S. Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biol Pharm Bull. 2011;34(12):1785-8. PMID:22130231
- ↑ Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006 May;7(5):359-71. PMID:16633338 doi:10.1038/nrm1911
- ↑ Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007 Oct;19(10):2003-12. Epub 2007 Jun 12. PMID:17658244 doi:10.1016/j.cellsig.2007.05.013
- ↑ Gallina P, Nohra G, Cioloca C, Meder JF, Roux FX. [Multiple cavernoma of delayed appearance] Neurochirurgie. 1994;40(5):322-5. PMID:7596453
- ↑ Shinkai A, Ito M, Anazawa H, Yamaguchi S, Shitara K, Shibuya M. Mapping of the sites involved in ligand association and dissociation at the extracellular domain of the kinase insert domain-containing receptor for vascular endothelial growth factor. J Biol Chem. 1998 Nov 20;273(47):31283-8. PMID:9813036
- ↑ Ruch C, Skiniotis G, Steinmetz MO, Walz T, Ballmer-Hofer K. Structure of a VEGF-VEGF receptor complex determined by electron microscopy. Nat Struct Mol Biol. 2007 Mar;14(3):249-50. Epub 2007 Feb 11. PMID:17293873 doi:10.1038/nsmb1202
- ↑ Matsumoto T, Bohman S, Dixelius J, Berge T, Dimberg A, Magnusson P, Wang L, Wikner C, Qi JH, Wernstedt C, Wu J, Bruheim S, Mugishima H, Mukhopadhyay D, Spurkland A, Claesson-Welsh L. VEGF receptor-2 Y951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis. EMBO J. 2005 Jul 6;24(13):2342-53. Epub 2005 Jun 16. PMID:15962004 doi:10.1038/sj.emboj.7600709
- ↑ Kendall RL, Rutledge RZ, Mao X, Tebben AJ, Hungate RW, Thomas KA. Vascular endothelial growth factor receptor KDR tyrosine kinase activity is increased by autophosphorylation of two activation loop tyrosine residues. J Biol Chem. 1999 Mar 5;274(10):6453-60. PMID:10037737
- ↑ Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002 Oct;2(10):795-803. PMID:12360282 doi:10.1038/nrc909
- ↑ Rosa DD, Ismael G, Lago LD, Awada A. Molecular-targeted therapies: lessons from years of clinical development. Cancer Treat Rev. 2008 Feb;34(1):61-80. Epub 2007 Sep 10. PMID:17826917 doi:10.1016/j.ctrv.2007.07.019
- ↑ Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001 Sep;7(9):987-9. PMID:11533692 doi:10.1038/nm0901-987