Sandbox Reserved 1717
From Proteopedia
(Difference between revisions)
| (10 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
<StructureSection load='' size='350' side='right' caption='Structure of Closed Vitamin K Epoxide Reductase (PDB entry [[6wv3]])' scene='90/904321/Closedconformation/2'> | <StructureSection load='' size='350' side='right' caption='Structure of Closed Vitamin K Epoxide Reductase (PDB entry [[6wv3]])' scene='90/904321/Closedconformation/2'> | ||
== Introduction == | == Introduction == | ||
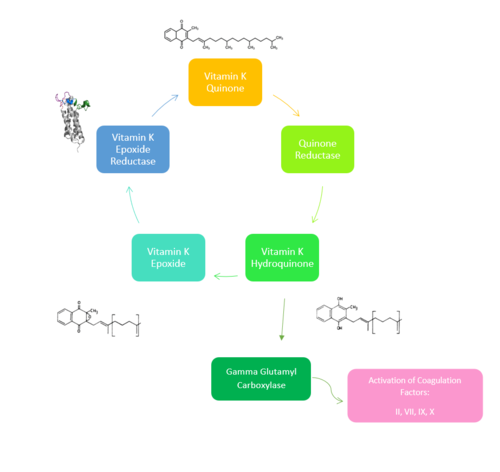
| - | [[Image:NewVitaminKCycle.PNG| | + | [[Image:NewVitaminKCycle.PNG|500px|right|thumb|'''Figure 1. Overview of Vitamin K Cycle''': The cycle begins with [https://en.wikipedia.org/wiki/Vitamin_K Vitamin K Quinone]. Vitamin K Quinone is reduced by enzyme Quinone Reductase. This leaves Vitamin K Hydroquinone which can either lead to [https://en.wikipedia.org/wiki/Gamma-glutamyl_carboxylase Gamma Carboxylase]activity that will activate Blood Coagulation Factors II, VII, IX, and X. After this, Vitamin K Epoxide is left over. Vitamin K Epoxide is reduced by the enzyme Vitamin K Epoxide Reductase to reform Vitamin K Quinone. ]] |
<scene name='90/904321/Vitamin_k_epoxide_reductase/1'>Vitamin K Epoxide Reductase</scene> | <scene name='90/904321/Vitamin_k_epoxide_reductase/1'>Vitamin K Epoxide Reductase</scene> | ||
| - | [https://en.wikipedia.org/wiki/Vitamin_K_epoxide_reductase | + | [https://en.wikipedia.org/wiki/Vitamin_K_epoxide_reductase (VKOR)] is an endoplasmic membrane enzyme that generates the active form of Vitamin K to support blood coagulation. VKOR homologs are integral membrane thiol oxidoreductases [https://en.wikipedia.org/wiki/Thiol_oxidoreductase Thiol OxidoReductase] due to the function of VKOR being dependent on thiol residues and disulfide bonding. The Vitamin K Cycle and the VKOR enzyme specifically are common drug targets for thromboembolic diseases. This is because, as pictured, the vitamin K cycle is required to activate blood coagulant factors [https://en.wikipedia.org/wiki/Thrombin II], [https://en.wikipedia.org/wiki/Coagulation_factor_VII VII], [https://en.wikipedia.org/wiki/Factor_IX IX], and [https://en.wikipedia.org/wiki/Factor_X#:~:text=Factor%20X%2C%20also%20known%20by,vitamin%20K%20for%20its%20synthesis. X]. Coagulant factor activation promotes blood clotting, which in high amounts can be dangerous and cause thromboembolic diseases such as stroke, deep vein thrombosis, and/or pulmonary embolism. Vitamin K Epoxide Reductase is found and primarily synthesized in the liver. It is embedded in the membrane known as the endoplasmic reticulum. |
===Reaction=== | ===Reaction=== | ||
| Line 49: | Line 49: | ||
As mentioned above, Vitamin K epoxide is a part of the Vitamin K cycle and required for blood coagulation. In the cycle, VKOR reduces Vitamin K epoxide to Vitamin K Quinone, or the active form of Vitamin K. In this conversion, VKOR donates electrons to Vitamin K epoxide from the S-H of the active pair of cysteines, C132-C135. The mediated cysteine pair, C43-C51, has to be reduced for the transfer of electrons to the substrate to occur. | As mentioned above, Vitamin K epoxide is a part of the Vitamin K cycle and required for blood coagulation. In the cycle, VKOR reduces Vitamin K epoxide to Vitamin K Quinone, or the active form of Vitamin K. In this conversion, VKOR donates electrons to Vitamin K epoxide from the S-H of the active pair of cysteines, C132-C135. The mediated cysteine pair, C43-C51, has to be reduced for the transfer of electrons to the substrate to occur. | ||
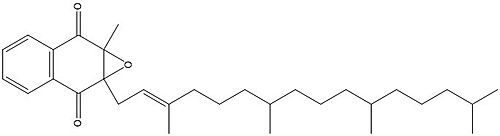
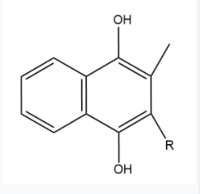
| - | Two other notable structures are Vitamin K Quinone (Fig. 5) and Vitamin K Hydroquinone (Fig. 6). Vitamin K Quinone is the product that is released after the reaction with Vitamin K Epoxide and VKOR. (Fig. | + | Two other notable structures are Vitamin K Quinone (Fig. 5) and Vitamin K Hydroquinone (Fig. 6). Vitamin K Quinone is the product that is released after the reaction with Vitamin K Epoxide and VKOR. (Fig. 1) |
[[Image:Vitaminkquinone.PNG|200 px|left|thumb|Figure 4. Vitamin K Quinone structure]] [[Image:Vitaminkhydroquinone.PNG|200 px|right|thumb|Figure 5. Vitamin K Hydroquinone structure]] | [[Image:Vitaminkquinone.PNG|200 px|left|thumb|Figure 4. Vitamin K Quinone structure]] [[Image:Vitaminkhydroquinone.PNG|200 px|right|thumb|Figure 5. Vitamin K Hydroquinone structure]] | ||
| Line 55: | Line 55: | ||
=== Binding === | === Binding === | ||
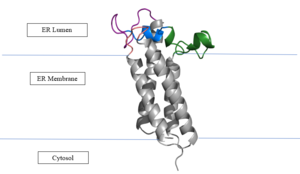
| - | In its resting state, VKOR is in its <scene name='90/904322/Open_conformation/ | + | In its resting state, VKOR is in its <scene name='90/904322/Open_conformation/2'>open conformation</scene>. The Vitamin K epoxide enters through the <scene name='90/904322/Tunnel/7'>isoprenyl-chain tunnel</scene>. The tunnel is located between <scene name='90/904322/Tunnel/8'>TM2 and TM3</scene>.<ref name="Li">PMID:20110994</ref> The carbonyls on the VK epoxide bind to <scene name='90/904322/Vko_binding/2'>Asn80 and Tyr139</scene> on VKOR. With Vitamin K epoxide bound, the conformation transitions from open to closed, where the catalytic process will begin. |
| - | + | ||
| - | + | ||
| - | + | ||
== Warfarin == | == Warfarin == | ||
[https://en.wikipedia.org/wiki/Warfarin Warfarin] is the most common [https://en.wikipedia.org/wiki/Vitamin_K_antagonist Vitamin K antagonist (VKA)]. Warfarin is a competitive inhibitor, taking the place of Vitamin K Epoxide (VKO) in the active site of Vitamin K Epoxide Reductase (VKOR). When warfarin binds in the active site, it causes VKOR to go into the closed conformation. | [https://en.wikipedia.org/wiki/Warfarin Warfarin] is the most common [https://en.wikipedia.org/wiki/Vitamin_K_antagonist Vitamin K antagonist (VKA)]. Warfarin is a competitive inhibitor, taking the place of Vitamin K Epoxide (VKO) in the active site of Vitamin K Epoxide Reductase (VKOR). When warfarin binds in the active site, it causes VKOR to go into the closed conformation. | ||
| - | [[Image:warfarin.jpg|400 px| | + | [[Image:warfarin.jpg|400 px|left|thumb|Figure 6. Warfarin structure]] |
=== Binding === | === Binding === | ||
| - | Warfarin still forms Hydrogen bonds with <scene name='90/904322/ | + | Warfarin still forms Hydrogen bonds with <scene name='90/904322/Asn80_tyr139_warfarin/1'>Asn80 and Tyr139</scene>. The specific bonds are between Asn80 and the 2-ketone group of warfarin and Tyr139 with the 4-hydroxyl group of warfarin. The rest of the pocket is hydrophobic interactions. The H bonds are necessary for the recognition of the ligand in the binding site of VKOR. |
| + | |||
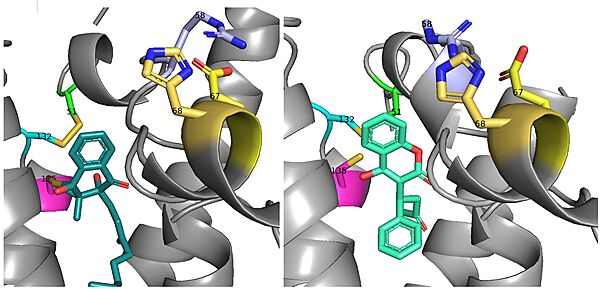
| + | There is a slight difference in the way in which warfarin binds compared to VKO. Warfarin binds are a slightly different angle (Fig.7). This creates a difference in how the cap loop and anchor domain interact, and that noticeable difference is with <scene name='90/904322/Arg58/4'>Arg58</scene>. With VKO, Arg58, located in the cap loop, directly interacts with <scene name='90/904322/Arg58_vko/5'>Glu67</scene> when VKO is bound. When warfarin binds, Arg58 is found inserted between <scene name='90/904322/Arg58_warfarin/2'>Glu67 and His68</scene> of the anchor domain.<ref name=”Liu”>PMID:33154105</ref> | ||
| + | [[Image:VKO and Warfarin binding.jpg|600 px|right|thumb|Figure 6. The slight angle change in which VKO(left) and warfarin(right) bind. The location of the cap domain and how it differs between each is apparent.]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| - | There is a slight difference in the way in which warfarin binds compared to VKO. Warfarin binds are a slightly different angle. This creates a difference in how the cap loop and anchor domain interact, and that noticeable difference is with <scene name='90/904322/Arg58/4'>Arg58</scene>. With VKO, Arg58, located in the cap loop, directly interacts with <scene name='90/904322/Arg58_vko/5'>Glu67</scene> when VKO is bound. When warfarin binds, Arg58 is found inserted between<scene name='90/904322/Arg58_warfarin/2'>Glu67 and His68</scene> of the anchor domain. | ||
=== Disease === | === Disease === | ||
[https://en.wikipedia.org/wiki/Vitamin_K_antagonist#:~:text=Vitamin%20K%20antagonists%20(VKA)%20are,the%20recycling%20of%20vitamin%20K. Vitamin K Antagonists] play a big role in the treatment of thromboembolic diseases, like a stroke or heart attack.<ref name="Goy">PMID:23034830</ref> [https://en.wikipedia.org/wiki/Warfarin Warfarin] is the most common medication for this treatment, acting as a blood thinner. Warfarin binding in VKOR overall prevents the triggering of coagulation factors that form blood clots. | [https://en.wikipedia.org/wiki/Vitamin_K_antagonist#:~:text=Vitamin%20K%20antagonists%20(VKA)%20are,the%20recycling%20of%20vitamin%20K. Vitamin K Antagonists] play a big role in the treatment of thromboembolic diseases, like a stroke or heart attack.<ref name="Goy">PMID:23034830</ref> [https://en.wikipedia.org/wiki/Warfarin Warfarin] is the most common medication for this treatment, acting as a blood thinner. Warfarin binding in VKOR overall prevents the triggering of coagulation factors that form blood clots. | ||
| + | |||
| + | |||
| + | |||
| + | |||
==References== | ==References== | ||
<references/> | <references/> | ||
Current revision
| |||||||||||