Sandbox Reserved 1706
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
==Introduction== | ==Introduction== | ||
| - | [https://en.wikipedia.org/wiki/Neurofibromatosis_type_I Neurofibromatosis Type 1] is a genetic disorder caused by mutations in the tumor suppressor gene NF1 that codes for the [https://en.wikipedia.org/wiki/GTPase-activating_protein GTPase-activating protein] neurofibromin.<ref name="Bergoug"> DOI:10.3390/cells9112365</ref> Neurofibromin is closely involved in signaling pathways such as [https://en.wikipedia.org/wiki/MAPK/ERK_pathway MAPK/ERK], [https://en.wikipedia.org/wiki/PI3K/AKT/mTOR_pathway P13K/AKT/mTOR], and other cell signaling pathways that use [https://en.wikipedia.org/wiki/Ras_GTPase Ras] <ref name="Bergoug"> DOI:10.3390/cells9112365</ref>. Decreased activity of neurofibromin due to mutation can lead to tumor growth along nerves. As it is ubiquitous expression, NF1 misregulation can cause systemic tumor growth. Neurofibromin is localized to the [https://en.wikipedia.org/wiki/Cytosol cytosol] but is recruited to the [https://en.wikipedia.org/wiki/Cell_membrane plasma membrane] to inactivate [https://en.wikipedia.org/wiki/Ras_GTPase Ras]. The structure of neurofibromin was determined by high-resolution single particle [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy cryo-EM]. These structures illustrated the domain architecture and conformational changes in neurofibromin, controlling Ras binding and inactivation. | + | [https://en.wikipedia.org/wiki/Neurofibromatosis_type_I Neurofibromatosis Type 1] is a genetic disorder caused by mutations in the tumor suppressor gene NF1 that codes for the [https://en.wikipedia.org/wiki/GTPase-activating_protein GTPase-activating protein] neurofibromin.<ref name="Bergoug"> DOI:10.3390/cells9112365</ref> Neurofibromin is closely involved in signaling pathways such as [https://en.wikipedia.org/wiki/MAPK/ERK_pathway MAPK/ERK], [https://en.wikipedia.org/wiki/PI3K/AKT/mTOR_pathway P13K/AKT/mTOR], and other cell signaling pathways that use [https://en.wikipedia.org/wiki/Ras_GTPase Ras] <ref name="Bergoug"> DOI:10.3390/cells9112365</ref>. Decreased activity of neurofibromin due to mutation can lead to tumor growth along nerves. As it is ubiquitous expression, NF1 misregulation can cause systemic tumor growth. Neurofibromin is localized to the [https://en.wikipedia.org/wiki/Cytosol cytosol] but is recruited to the [https://en.wikipedia.org/wiki/Cell_membrane plasma membrane] to inactivate [https://en.wikipedia.org/wiki/Ras_GTPase Ras]. The structure of neurofibromin was determined by high-resolution single particle [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy cryo-EM].<ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> These structures illustrated the domain architecture and conformational changes in neurofibromin, controlling Ras binding and inactivation. <ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref> |
==Function== | ==Function== | ||
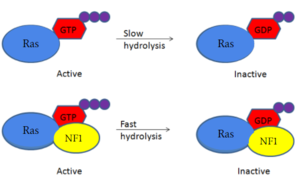
| - | Neurofibromin is a [https://en.wikipedia.org/wiki/GTPase-activating_protein GTPase-activating protein] that binds to [https://en.wikipedia.org/wiki/Ras_GTPase Ras], a [https://en.wikipedia.org/wiki/GTPase GTPase], to increase its inherent hydrolysis of GTP to GDP (Figure 1). This inactivates the cell signaling of Ras until reactivated by [https://en.wikipedia.org/wiki/Guanosine_triphosphate GTP] exchange. Neurofibromin has two structural conformations, the open and closed conformation. Neurofibromin only binds to Ras in its open conformation. | + | Neurofibromin is a [https://en.wikipedia.org/wiki/GTPase-activating_protein GTPase-activating protein] that binds to [https://en.wikipedia.org/wiki/Ras_GTPase Ras], a [https://en.wikipedia.org/wiki/GTPase GTPase], to increase its inherent hydrolysis of GTP to GDP (Figure 1).<ref name="Bourne"> DOI:10.1038/39470</ref> This inactivates the cell signaling of Ras until reactivated by [https://en.wikipedia.org/wiki/Guanosine_triphosphate GTP] exchange. Neurofibromin has two structural conformations, the open and closed conformation. Neurofibromin only binds to Ras in its open conformation. <ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> [[Image:RasNeurofibrominMech.PNG|300px|right|thumb|Figure 1: Shifted rate of of Ras GTP hydrolysis when bound to neurofibromin. The speed of GTP hydrolysis is significantly increased when bound to neurofibromin. Ras is inactive when bound to GDP and active when bound to GTP ]] |
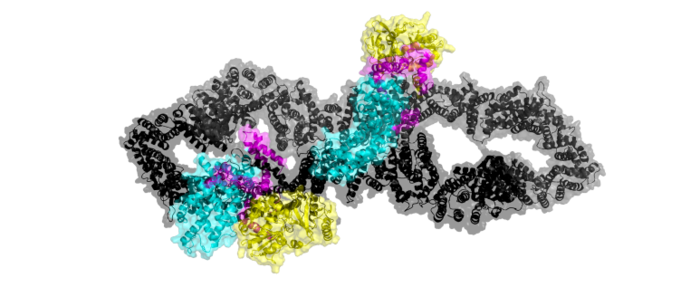
==Structure== | ==Structure== | ||
| Line 33: | Line 33: | ||
====Closed conformation==== | ====Closed conformation==== | ||
| - | Ras is unable to bind to the GRD binding site when both of the neurofibromin protomers are in the <scene name='90/904311/Closed_conformation/8'>closed conformation</scene>. In the closed conformation, one protomer has its domains shifted by a 130° rotation of three separate conformational change linkers. That rotation places Arg1276 in an orientation where binding of Y32 of Ras is sterically hindered by E31. (<scene name='90/904311/Closed_arg/6'>Arg1276, Y32, and E31 interaction</scene> | + | Ras is unable to bind to the GRD binding site when both of the neurofibromin protomers are in the <scene name='90/904311/Closed_conformation/8'>closed conformation</scene>. In the closed conformation, one protomer has its domains shifted by a 130° rotation of three separate conformational change linkers.<ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> That rotation places Arg1276 in an orientation where binding of Y32 of Ras is sterically hindered by E31.<ref name="Bourne"> DOI:10.1038/39470</ref> (<scene name='90/904311/Closed_arg/6'>Arg1276, Y32, and E31 interaction</scene> |
<jmol> | <jmol> | ||
<jmolButton> | <jmolButton> | ||
<script>moveto 1.0 { 347 936 -57 112.47} 2508.34 0.0 0.0 {366.9273333333333 357.0333333333333 434.887} 189.81815871562094 {0 0 0} 0 0 0 3.0 0.0 0.0</script> <text>🔎</text> | <script>moveto 1.0 { 347 936 -57 112.47} 2508.34 0.0 0.0 {366.9273333333333 357.0333333333333 434.887} 189.81815871562094 {0 0 0} 0 0 0 3.0 0.0 0.0</script> <text>🔎</text> | ||
</jmolButton> | </jmolButton> | ||
| - | </jmol>) Ras binding to the GRD site is inhibited by steric occlusion from the N-HEAT/ARM. The closed conformation can exist naturally without any form of stabilization but exists in a natural equilibrium with the open conformation. | + | </jmol>) Ras binding to the GRD site is inhibited by steric occlusion from the N-HEAT/ARM. The closed conformation can exist naturally without any form of stabilization but exists in a natural equilibrium with the open conformation. |
=====Zinc Stabilized===== | =====Zinc Stabilized===== | ||
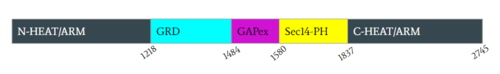
| - | The closed conformation is stabilized by a zinc ion that prevents the shift back to the open conformation. Zinc binding is chelated by three residues (<scene name='90/904311/Zinc_binding_site/11'>Cys1032, His1558, His1576</scene>). These three residues are contributed by the N-HEAT domain (Cys1032) and the GAPex-subdomain (His1558 and His1576). Zinc stabilization keeps Neurofibromin in the closed conformation, inhibiting Ras binding.<ref name="Bourne"> DOI:10.1038/39470 | + | The closed conformation is stabilized by a zinc ion that prevents the shift back to the open conformation. Zinc binding is chelated by three residues (<scene name='90/904311/Zinc_binding_site/11'>Cys1032, His1558, His1576</scene>). These three residues are contributed by the N-HEAT domain (Cys1032) and the GAPex-subdomain (His1558 and His1576). Zinc stabilization keeps Neurofibromin in the closed conformation, inhibiting Ras binding.<ref name="Bourne"> DOI:10.1038/39470</ref><ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> |
====Open conformation==== | ====Open conformation==== | ||
| - | In the <scene name='90/904311/Open_conformation/14'> open conformation</scene>, one protomer is shifted to allow Ras binding, while the other protomer remains in the closed conformation. In the open conformation one protomer is rotated 90°, facilitating binding between Y32 of RAS and Arg1276. (<scene name='90/904311/Arg_1276_mi/2'>Y32 and Arg1276 interaction</scene> | + | In the <scene name='90/904311/Open_conformation/14'> open conformation</scene>, one protomer is shifted to allow Ras binding, while the other protomer remains in the closed conformation. In the open conformation one protomer is rotated 90°, facilitating binding between Y32 of RAS and Arg1276.<ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref><ref name="Bourne"> DOI:10.1038/39470</ref> (<scene name='90/904311/Arg_1276_mi/2'>Y32 and Arg1276 interaction</scene> |
<jmol> | <jmol> | ||
<jmolButton> | <jmolButton> | ||
| Line 50: | Line 50: | ||
</jmolButton> | </jmolButton> | ||
</jmol>) | </jmol>) | ||
| - | in the GRD site. To allow Ras binding, the GRD and Sec14-PH domains are reoriented away from one another and the GRD site is accessible for Ras binding. This conformational change to the open conformation is driven by rearrangement of three separate linkers (L1, L2, L3). | + | in the GRD site. To allow Ras binding, the GRD and Sec14-PH domains are reoriented away from one another and the GRD site is accessible for Ras binding. This conformational change to the open conformation is driven by rearrangement of three separate linkers (L1, L2, L3). |
====Conformational Change Linkers==== | ====Conformational Change Linkers==== | ||
| Line 64: | Line 64: | ||
</jmolButton> | </jmolButton> | ||
</jmol>). Linker 1 (L1) consists of a loop connected by two helices from L1173-M1215 and is the main contributor in rotation of the GRD domain. The rotation of L1 to the open conformation causes N-HEAT/ARM [https://en.wikipedia.org/wiki/Alpha_helix α helix] 48 and GRD helix 49 to extend out, aligning to form a hinge point at G1190.<ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | </jmol>). Linker 1 (L1) consists of a loop connected by two helices from L1173-M1215 and is the main contributor in rotation of the GRD domain. The rotation of L1 to the open conformation causes N-HEAT/ARM [https://en.wikipedia.org/wiki/Alpha_helix α helix] 48 and GRD helix 49 to extend out, aligning to form a hinge point at G1190.<ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> | ||
| - | The GRD relocation is assisted by Sec14-PH relocation, which is initiated by Linker 3(L3) from Q1835 to G1852. Movement of L3 is further supported by rearrangement of the proline rich section of the C-HEAT/ARM. L1 and L3 also move closer to each other in the open conformation initiating rearrangement of the GRD and Sec14-PH domain. Linker 2 (L2) consists of residues G1547-T1565 and begins at helix 63, the final helix of the GRD site, and connects into the short loop of [https://en.wikipedia.org/wiki/Alpha_helix α helix] 65 of the Sec14-PH domain and also assists in shifting the Sec14-PH away from the GRD site. The combination of these three linkers are largely responsible for the conformational shift of the closed and open conformation.<ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref> | + | The GRD relocation is assisted by Sec14-PH relocation, which is initiated by Linker 3(L3) from Q1835 to G1852. Movement of L3 is further supported by rearrangement of the proline rich section of the C-HEAT/ARM. L1 and L3 also move closer to each other in the open conformation initiating rearrangement of the GRD and Sec14-PH domain. Linker 2 (L2) consists of residues G1547-T1565 and begins at helix 63, the final helix of the GRD site, and connects into the short loop of [https://en.wikipedia.org/wiki/Alpha_helix α helix] 65 of the Sec14-PH domain and also assists in shifting the Sec14-PH away from the GRD site.<ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> The combination of these three linkers are largely responsible for the conformational shift of the closed and open conformation.<ref name="Lupton"> DOI:10.1038/s41594-021-00687-2</ref> |
| - | + | ||
==Ras Binding== | ==Ras Binding== | ||
Arg1276 is the critical residue within the GRD site for Ras activation. When <scene name='90/904311/Arg_1276_open/13'>Arg1276 is in the open conformation</scene> | Arg1276 is the critical residue within the GRD site for Ras activation. When <scene name='90/904311/Arg_1276_open/13'>Arg1276 is in the open conformation</scene> | ||
| Line 72: | Line 71: | ||
<script>moveto 1.0 { -776 433 -458 146.98} 7209.11 0.0 0.0 {361.5375833333334 402.0207083333333 360.750125} 190.41144084626742 {0 0 0} 0 0 0 3.0 0.0 0.0</script> <text>🔎</text> | <script>moveto 1.0 { -776 433 -458 146.98} 7209.11 0.0 0.0 {361.5375833333334 402.0207083333333 360.750125} 190.41144084626742 {0 0 0} 0 0 0 3.0 0.0 0.0</script> <text>🔎</text> | ||
</jmolButton> | </jmolButton> | ||
| - | </jmol>, the arginine finger binds to the backbone γ-carbon of Y32 in Ras to assist in eventual hydrolysis of GTP. When <scene name='90/904311/Closed_zoom/10'>Arg1276 is in the closed conformation</scene> | + | </jmol>, the arginine finger binds to the backbone γ-carbon of Y32 in Ras to assist in eventual hydrolysis of GTP.<ref name="Bourne"> DOI:10.1038/39470</ref> When <scene name='90/904311/Closed_zoom/10'>Arg1276 is in the closed conformation</scene> |
<jmol> | <jmol> | ||
<jmolButton> | <jmolButton> | ||
<script>moveto 1.0 { -816 -263 -515 73.84} 7673.3 0.0 0.0 {369.48317647058826 352.12982352941174 432.1719411764706} 186.8074765770222 {0 0 0} 0 0 0 3.0 0.0 0.0</script> <text>🔎</text> | <script>moveto 1.0 { -816 -263 -515 73.84} 7673.3 0.0 0.0 {369.48317647058826 352.12982352941174 432.1719411764706} 186.8074765770222 {0 0 0} 0 0 0 3.0 0.0 0.0</script> <text>🔎</text> | ||
</jmolButton> | </jmolButton> | ||
| - | </jmol> the interaction between Y32 and GRD (R1276) is blocked by E31. Removal of the inhibition of E31 from Ras by R1276 from Neurofibromin allows for the normal function of neurofibromin in the rapid rate increase of GTP hydrolysis upon Ras binding.<ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> Mutations to the arginine finger slow GTPase activating reaction of neurofibromin.<ref name="Bourne"> DOI:10.1038/39470 | + | </jmol> the interaction between Y32 and GRD (R1276) is blocked by E31. Removal of the inhibition of E31 from Ras by R1276 from Neurofibromin allows for the normal function of neurofibromin in the rapid rate increase of GTP hydrolysis upon Ras binding.<ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> Mutations to the arginine finger slow GTPase activating reaction of neurofibromin.<ref name="Bourne"> DOI:10.1038/39470</ref> |
==SPRED 1== | ==SPRED 1== | ||
| - | <scene name='90/904311/Spred1_w_grd/2'>SPRED 1</scene> is another peripheral protein that interacts with the GRD domain of Neurofibromin. SPRED 1 recruits Neurofibromin from the cytosol to the plasma membrane to interact with Ras. Binding of SPRED 1 can occur in either the open or closed conformation and causes a structural rearrangement of the GRD and GAPex domains that has yet to be structuralized. | + | <scene name='90/904311/Spred1_w_grd/2'>SPRED 1</scene> is another peripheral protein that interacts with the GRD domain of Neurofibromin. SPRED 1 recruits Neurofibromin from the cytosol to the plasma membrane to interact with Ras. Binding of SPRED 1 can occur in either the open or closed conformation and causes a structural rearrangement of the GRD and GAPex domains that has yet to be structuralized.<ref name="Naschberger"> DOI:10.1038/s41586-021-04024-x</ref> |
==Clinical Relevance== | ==Clinical Relevance== | ||
[https://en.wikipedia.org/wiki/Germline_mutation Germline mutations] are common in NF1 and often cause genetic tumor syndrome through misregulation of the Ras signaling pathway. <ref name="Kioro"> DOI:10.1038/labinvest.2016.142</ref> [https://en.wikipedia.org/wiki/Somatic_mutation Somatic mutations] among NF1 are also extremely common. In germline mutations and some somatic mutations of NF1, tumors develop along the deep epidermis layer of the skin. Understanding how mutations affect the structure and function of neurofibromin will allow for advancements in treatment. | [https://en.wikipedia.org/wiki/Germline_mutation Germline mutations] are common in NF1 and often cause genetic tumor syndrome through misregulation of the Ras signaling pathway. <ref name="Kioro"> DOI:10.1038/labinvest.2016.142</ref> [https://en.wikipedia.org/wiki/Somatic_mutation Somatic mutations] among NF1 are also extremely common. In germline mutations and some somatic mutations of NF1, tumors develop along the deep epidermis layer of the skin. Understanding how mutations affect the structure and function of neurofibromin will allow for advancements in treatment. | ||
| - | <ref name="Bergoug"> DOI:10.3390/cells9112365 | + | <ref name="Bergoug"> DOI:10.3390/cells9112365</ref><ref name="Sabatini"> DOI:10.1007/s11940-015-0355-4</ref> |
==References== | ==References== | ||
<references/> | <references/> | ||
Current revision
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
Neurofibromin 1
| |||||||||||