Sandbox Reserved 1722
From Proteopedia
(Difference between revisions)
| (10 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | {{Template:CH462_Biochemistry_II_2022}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
== '''''Human Itch Mas-Related G-Protein Coupled Receptor''''' == | == '''''Human Itch Mas-Related G-Protein Coupled Receptor''''' == | ||
| Line 24: | Line 23: | ||
==== ''Toggle Switch'' ==== | ==== ''Toggle Switch'' ==== | ||
| - | In β2AR, and other Class A GPCRs, a “toggle switch” of <scene name='90/904327/B2artoggleswitchyes/ | + | In β2AR, and other Class A GPCRs, a “toggle switch” of <scene name='90/904327/B2artoggleswitchyes/8'>Trp-286</scene> limits the proximity of the TM helices as tryptophan sterically occludes tight interaction. This results in a deep binding pocket for ligand binding. In contrast, in MRGPRX2 Trp-286 is replaced by <scene name='90/904327/Toggle_switch_gly_pt_2/3'>Gly-236</scene> <ref name="Cao"/> <ref name="Yang"/>. Glycine is a much smaller amino acid and thus allows the helices to close the base of the binding pocket. This causes MRGPRX2 to have a much shallower binding site and allows more promiscuous ligand binding. This can be seen in a shorter distance from the toggle switch to the ligand in β2AR (0.573 nm) compared to MRGPRX2 (1.389 nm). |
==== ''Disulfide bonds'' ==== | ==== ''Disulfide bonds'' ==== | ||
| - | In common Class A GPCRs the disulfide bond associated with the initiation of signal transduction is located on the extracellular domain of the 7 transmembrane helices <ref name="Zhang">PMID: 26467290</ref>. The close proximity of the disulfide bond to the ligand binding site, allows the receptor to know that it is being bound and initiate the signal through the rest of the receptor. The <scene name='90/904328/B2ardisulfidebond1_pt_3/1'>disulfide bond of β2AR</scene>, a well-studied Class A GPCR, occurs between transmembrane three (TM3) Cys-106 and an extracellular loop (EL) Cys-191. This loop crosses through the middle of the extracellular domain, creating a barrier for bulkier substrates. The <scene name='90/904327/7tm_domain_pt_6/1'>disulfide bond of MRGPRX2</scene> is located between (TM4) Cys-168 and (TM5) Cys-180. This is a TM to TM disulfide bond as compared to a TM to EL disulfide bond seen in typical Class A GPCRs. This lack of interaction with the extracellular loop seen in MRGPRX2 causes the extracellular loop to flip on top of the TM4 and TM5 resulting in an open space for larger substrates to be able to interact with the receptor. | + | In common Class A GPCRs, the disulfide bond associated with the initiation of signal transduction is located on the extracellular domain of the 7 transmembrane helices <ref name="Zhang">PMID: 26467290</ref>. The close proximity of the disulfide bond to the ligand binding site, allows the receptor to know that it is being bound and initiate the signal through the rest of the receptor. The <scene name='90/904328/B2ardisulfidebond1_pt_3/1'>disulfide bond of β2AR</scene>, a well-studied Class A GPCR, occurs between transmembrane three (TM3) Cys-106 and an extracellular loop (EL) Cys-191. This loop crosses through the middle of the extracellular domain, creating a barrier for bulkier substrates. The <scene name='90/904327/7tm_domain_pt_6/1'>disulfide bond of MRGPRX2</scene> is located between (TM4) Cys-168 and (TM5) Cys-180. This is a TM to TM disulfide bond as compared to a TM to EL disulfide bond seen in typical Class A GPCRs. This lack of interaction with the extracellular loop seen in MRGPRX2 causes the extracellular loop to flip on top of the TM4 and TM5 resulting in an open space for larger substrates to be able to interact with the receptor. |
==== ''PIF Motif'' ==== | ==== ''PIF Motif'' ==== | ||
| Line 34: | Line 33: | ||
MRGPRX2 also differs from typical Class A GPCRs based on substitutions in the PIF/connector motif, which acts as a microswitch. PIF plays a role in connecting the binding pocket to conformational rearrangements required for receptor activation<ref name="Schonegge">Schonegge, Anne-Marie, et al. "Evolutionary action and structural basis of the allosteric switch controlling β2AR functional selectivity." Nature, Nature Publishing Group, 18 December 2017, https://www.nature.com/articles/s41467-017-02257-x</ref>. The PIF motif is located towards the base of the TM domains. In a Class A GPCR, like <scene name='90/904327/B2arpif_pt_2/1'>β2AR, the PIF motif</scene> consists of Phe-211, Ile-121, and Phe-282 on TM domains 5, 3, and 6, respectively. However, for <scene name='90/904327/Mxpifnolabel_pt_3/1'>MRGPRX2, this PIF motif</scene> is replaced with Leu-194 on TM5, Leu-117 on TM3, and Phe-232 on TM6. These modifications result in tighter packing around the base of the receptor, indicating slightly different interactions with the intracellular [https://en.wikipedia.org/wiki/G_protein G-proteins]. | MRGPRX2 also differs from typical Class A GPCRs based on substitutions in the PIF/connector motif, which acts as a microswitch. PIF plays a role in connecting the binding pocket to conformational rearrangements required for receptor activation<ref name="Schonegge">Schonegge, Anne-Marie, et al. "Evolutionary action and structural basis of the allosteric switch controlling β2AR functional selectivity." Nature, Nature Publishing Group, 18 December 2017, https://www.nature.com/articles/s41467-017-02257-x</ref>. The PIF motif is located towards the base of the TM domains. In a Class A GPCR, like <scene name='90/904327/B2arpif_pt_2/1'>β2AR, the PIF motif</scene> consists of Phe-211, Ile-121, and Phe-282 on TM domains 5, 3, and 6, respectively. However, for <scene name='90/904327/Mxpifnolabel_pt_3/1'>MRGPRX2, this PIF motif</scene> is replaced with Leu-194 on TM5, Leu-117 on TM3, and Phe-232 on TM6. These modifications result in tighter packing around the base of the receptor, indicating slightly different interactions with the intracellular [https://en.wikipedia.org/wiki/G_protein G-proteins]. | ||
| - | [[Image:LabeledDRY.PNG|250px|right|thumb|'''Figure 2''': DRY Motif of MRGPRX2 <ref name="Yang">Yang, Fan, et al. "Structure, function and pharmacology of human itch receptor complexes." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04077-y</ref> | + | [[Image:LabeledDRY.PNG|250px|right|thumb|'''Figure 2''': DRY Motif of MRGPRX2 [https://www.rcsb.org/structure/7S8L 7S8L] <ref name="Yang">Yang, Fan, et al. "Structure, function and pharmacology of human itch receptor complexes." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04077-y</ref>]] |
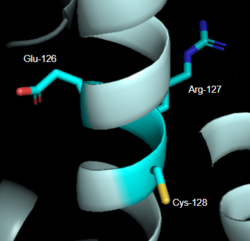
==== ''DRY Motif'' ==== | ==== ''DRY Motif'' ==== | ||
| - | The DRY motif is a proton microswitch that is located near the G-protein binding site C-terminal on TM3 <ref name="Schonegge"/>. It acts as lock when the GPCR is not being activated, preventing unnecessary activation of the G-proteins <ref name="Zhou"/>. This motif is conserved in typical Class A GPCRS however, in MRGPRX2 it is only partially conserved. The arginine is conserved, while the aspartate is replaced by a glutamate and the tyrosine is replaced by a cysteine <ref name="Yang"/> <ref name="Sandoval">Sandoval, A., et al. "The Molecular Switching Mechanism at the Conserved D(E)RY Motif in Class-A GPCRs." Biophysical journal, 111(1), 79-89. https://doi.org/10.1016/j.bpj.2016.06.004 </ref> (Figure 2). The replacement of tyrosine for cysteine results in the helices coming closer together, creating a shallower binding pocket<ref name="Zhou"/>. | + | The DRY motif is a proton microswitch that is located near the G-protein binding site C-terminal on TM3 <ref name="Schonegge"/>. It acts as lock when the GPCR is not being activated, preventing unnecessary activation of the G-proteins <ref name="Zhou"/>. This motif is conserved in typical Class A GPCRS however, in MRGPRX2 it is only partially conserved. The arginine is conserved, while the aspartate is replaced by a glutamate and the tyrosine is replaced by a cysteine <ref name="Yang"/> <ref name="Sandoval">Sandoval, A., et al. "The Molecular Switching Mechanism at the Conserved D(E)RY Motif in Class-A GPCRs." Biophysical journal, 111(1), 79-89. https://doi.org/10.1016/j.bpj.2016.06.004 </ref> (Figure 2). The replacement of tyrosine for cysteine results in the helices coming closer together, creating a shallower binding pocket <ref name="Zhou"/>. |
==== ''Sodium Binding'' ==== | ==== ''Sodium Binding'' ==== | ||
| - | The sodium site motif facilitates the conformational change of GPCR upon activation<ref name="Katritch">PMID: 24767681</ref>. A sodium molecule sits in the middle of the TM7 helices where it is stabilized by conserved residues aspartate (TM2), serine (TM2), and three water molecules. The sodium is able to make a salt bridge with the aspartate at this position. The sodium acts similar to a ball joint in which it allows for the TM helices be spread apart and induce larger conformational change upon binding. In MRGPRX2, this motif is only partially conserved. The aspartate (TM2) is conserved while the serine is replaced by a glycine <ref name="Yang"/> .This creates a less favorable environment for the stabilization of sodium due to serine being polar while glycine is nonpolar. Currently, in crystallization structures no sodium has been seen at this site. Thus making it inconclusive on whether it plays a role in the conformational change to activate G-proteins upon binding to the receptor <ref name="Yang"/> . | + | The sodium site motif facilitates the conformational change of GPCR upon activation <ref name="Katritch">PMID: 24767681</ref>. A sodium molecule sits in the middle of the TM7 helices where it is stabilized by conserved residues aspartate (TM2), serine (TM2), and three water molecules. The sodium is able to make a salt bridge with the aspartate at this position. The sodium acts similar to a ball joint in which it allows for the TM helices be spread apart and induce larger conformational change upon binding. In MRGPRX2, this motif is only partially conserved. The aspartate (TM2) is conserved while the serine is replaced by a glycine <ref name="Yang"/>. This creates a less favorable environment for the stabilization of sodium due to serine being polar while glycine is nonpolar. Currently, in crystallization structures no sodium has been seen at this site. Thus making it inconclusive on whether it plays a role in the conformational change to activate G-proteins upon binding to the receptor <ref name="Yang"/>. |
== MRGPRX2 Signaling Pathway == | == MRGPRX2 Signaling Pathway == | ||
| Line 53: | Line 52: | ||
==== Agonists ==== | ==== Agonists ==== | ||
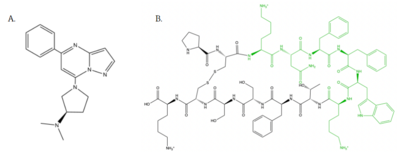
| - | [[Image:Agoniststogether.PNG |400px|right|thumb|'''Figure 4''': Structure of MRGPRX2 Agonists. (A) Structure of R-Zinc 3573. A cationic ligand selected for binding to MRGPRX2 <ref name="Yang">Yang, Fan, et al. "Structure, function and pharmacology of human itch receptor complexes." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04077-y</ref>.(B) Structure of Cortistatin-14 with resolved amino acids highlighted in green. These ligands were used as a probes for MRGPRX2 function and stabilization for structure determination <ref name="Yang">Yang, Fan, et al. "Structure, function and pharmacology of human itch receptor complexes." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04077-y</ref>.]] | + | [[Image:Agoniststogether.PNG |400px|right|thumb|'''Figure 4''': Structure of MRGPRX2 Agonists. (A) Structure of R-Zinc 3573. A cationic ligand selected for binding to MRGPRX2 <ref name="Yang">Yang, Fan, et al. "Structure, function and pharmacology of human itch receptor complexes." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04077-y</ref>. (B) Structure of Cortistatin-14 with resolved amino acids highlighted in green. These ligands were used as a probes for MRGPRX2 function and stabilization for structure determination <ref name="Yang">Yang, Fan, et al. "Structure, function and pharmacology of human itch receptor complexes." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04077-y</ref>.]] |
===== Small Molecule ===== | ===== Small Molecule ===== | ||
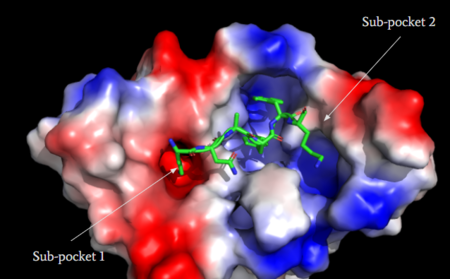
| - | <scene name='90/904328/Zinc/4'>(R)-Zinc-3573</scene> is a cation ligand that selectively binds to MRGPRX2 (Figure | + | <scene name='90/904328/Zinc/4'>(R)-Zinc-3573</scene> is a cation ligand that selectively binds to MRGPRX2 (Figure 4A). Its N-dimethyl is inserted into subpocket 1 of the binding cavity with aromatic amino acid residues Phe-170, Trp-243, and Phe-244. In this <scene name='90/904328/Zizwithaa/6'>cavity</scene>, N-dimethyl group on the ligand makes ion pairs with Asp-184 and Glu-164. The ligand is then stabilized by stacking its ring with Trp-248 and the Cys-168 to Cys-180 disulfide bond <ref name="Yang">Yang, Fan, et al. "Structure, function and pharmacology of human itch receptor complexes." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04077-y</ref>. |
===== Peptide ===== | ===== Peptide ===== | ||
| - | <scene name='90/904328/Overview_x2_c_pt_3/4'>Cortistatin-14</scene> is one of the peptide ligands that binds to MRGPRX2 (Figure | + | <scene name='90/904328/Overview_x2_c_pt_3/4'>Cortistatin-14</scene> is one of the peptide ligands that binds to MRGPRX2 (Figure 4B). Cortistatin-14 interacts with the binding pocket through an <scene name='90/904328/Zic14_pt_3/5'>electrostatic</scene> interaction in sub-pocket 1 between Lys-3 on the peptide and Glu-164 and Asp-184 on MRGPRX2 <ref name="Cao"/>. Additionally, there are hydrophobic interactions in sub-pocket 2 between the peptide and the binding pocket due to the large hydrophobic amino acids on Cortistatin-14. |
| - | + | ||
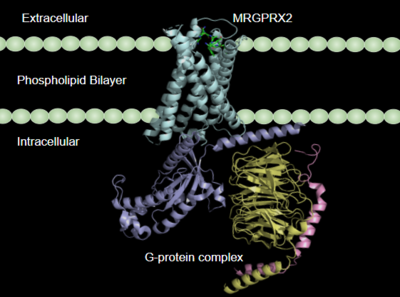
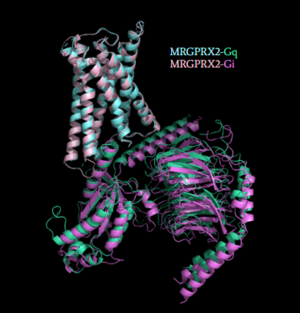
=== 2. MRGPRX2 interaction with G-Protein === | === 2. MRGPRX2 interaction with G-Protein === | ||
| Line 77: | Line 75: | ||
== Clinical Relevance == | == Clinical Relevance == | ||
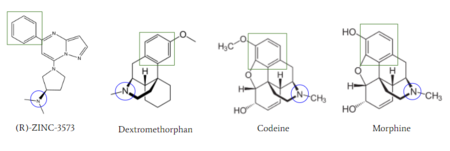
| - | [[Image:Zinc_and_drugs_snip.PNG|450px|right|thumb|'''Figure 6''': Structures of (R)-Zinc-3573, Dextromethorphan, Morphine, and Codeine. The blue circles indicate conserved basic N-dimethyl group and the green squares show the conserved benzene rings | + | [[Image:Zinc_and_drugs_snip.PNG|450px|right|thumb|'''Figure 6''': Structures of (R)-Zinc-3573, Dextromethorphan, Morphine, and Codeine. The blue circles indicate conserved basic N-dimethyl group and the green squares show the conserved benzene rings <ref name="Cao"/>.]] |
| - | MRGPRX2 activation is associated with chronic itching or anaphylaxis reactions, a common side effect of many prescribed medications | + | MRGPRX2 activation is associated with chronic itching or anaphylaxis reactions, a common side effect of many prescribed medications <ref name="Cao"/>. Among these drugs are opioids [https://en.wikipedia.org/wiki/Morphine morphine] and [https://en.wikipedia.org/wiki/Codeine codeine] and [https://en.wikipedia.org/wiki/Dextromethorphan dextromethorphan]. By analyzing the structures of these drugs, it can be seen that they contain chemical features similar to <scene name='90/904328/Zizwithaa/6'>agonist R-Zinc-3573</scene> (see Figure 6). They contain a conserved benzene ring that stabilizes them in binding pocket 1. Similarly, they contain an N-dimethyl group that would allow them to form key ionic bonds with residues Asp-184 and Glu-164. Lastly, these drugs have a similar shape and size to that of the agonist R-Zinc-3573 <ref name="Babina"> Babina, M., et al. "MRGPRX2 Is the Codeine Receptor of Human Skin Mast Cells: Desensitization through β-Arrestin and Lack of Correlation with the FcεRI Pathway." Journal of Investigative Dermatology, 141(6), 1286-1296. https://doi.org/10.1016/j.jid.2020.09.017</ref> (see Figure 6). These structural and chemical similarities indicate the possibility of a similar binding mechanism to previously discussed agonist, (R)-Zinc-3573. |
This receptor is shallow and is able to bind to a variety of drugs, creating a need for a solution to mediate the effects of MRGPRX2 activation. Currently, there is research for the development of small antagonists that will induce an anti-inflammatory effect by preventing [https://en.wikipedia.org/wiki/Immunoglobulin_E IgE-dependent] mast cell degranulation <ref name="McNeil">McNeil, B. D., et al. "MRGPRX2 and Adverse Drug Reactions." Frontier Immunology, 06 August 2021, https://www.frontiersin.org/articles/10.3389/fimmu.2021.676354/full</ref>. There are two antagonists that have been adapted from being antagonists to another Class A GPCR, [https://en.wikipedia.org/wiki/NK1_receptor_antagonist neurokinin-1 receptor (NK-1R)] <ref name="Ogasawara">Ogasawara, H., et al. "Novel MRGPRX2 antagonists inhibit IgE-independent activation of human umbilical cord blood-derived mast cells." Journal of Leukocyte Biology, 12 July 2019, https://jlb.onlinelibrary.wiley.com/doi/10.1002/JLB.2AB1018-405R</ref>. Through the development of this research, these adverse side effects can be relieved and improve patient satisfaction. | This receptor is shallow and is able to bind to a variety of drugs, creating a need for a solution to mediate the effects of MRGPRX2 activation. Currently, there is research for the development of small antagonists that will induce an anti-inflammatory effect by preventing [https://en.wikipedia.org/wiki/Immunoglobulin_E IgE-dependent] mast cell degranulation <ref name="McNeil">McNeil, B. D., et al. "MRGPRX2 and Adverse Drug Reactions." Frontier Immunology, 06 August 2021, https://www.frontiersin.org/articles/10.3389/fimmu.2021.676354/full</ref>. There are two antagonists that have been adapted from being antagonists to another Class A GPCR, [https://en.wikipedia.org/wiki/NK1_receptor_antagonist neurokinin-1 receptor (NK-1R)] <ref name="Ogasawara">Ogasawara, H., et al. "Novel MRGPRX2 antagonists inhibit IgE-independent activation of human umbilical cord blood-derived mast cells." Journal of Leukocyte Biology, 12 July 2019, https://jlb.onlinelibrary.wiley.com/doi/10.1002/JLB.2AB1018-405R</ref>. Through the development of this research, these adverse side effects can be relieved and improve patient satisfaction. | ||
Current revision
Human Itch Mas-Related G-Protein Coupled Receptor
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Cao, Can, et al. "Structure, function and pharmacology of human itch GPCRs." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04126-6
- ↑ Thal, David M., et al. "Structural insights into G-protein-coupled receptor allostery." Nature, Nature Publishing Group, 04 July 2018, https://www.nature.com/articles/s41586-018-0259-z
- ↑ 3.0 3.1 Zhang D, Zhao Q, Wu B. Structural Studies of G Protein-Coupled Receptors. Mol Cells. 2015 Oct;38(10):836-42. doi: 10.14348/molcells.2015.0263. Epub 2015, Oct 15. PMID:26467290 doi:http://dx.doi.org/10.14348/molcells.2015.0263
- ↑ 4.0 4.1 Ramesh, Soliman, et al. (2015) "G-Protein Coupled Receptors (GPCRs): A Comprehensive Computational Perspective." Combinational Chemistry and High Throughout Screening, 18(4), 346-364, https://pubmed.ncbi.nlm.nih.gov/25747435/
- ↑ 5.0 5.1 5.2 5.3 Zhou Q, Yang D, Wu M, Guo Y, Guo W, Zhong L, Cai X, Dai A, Jang W, Shakhnovich EI, Liu ZJ, Stevens RC, Lambert NA, Babu MM, Wang MW, Zhao S. Common activation mechanism of class A GPCRs. Elife. 2019 Dec 19;8. pii: 50279. doi: 10.7554/eLife.50279. PMID:31855179 doi:http://dx.doi.org/10.7554/eLife.50279
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 Yang, Fan, et al. "Structure, function and pharmacology of human itch receptor complexes." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04077-y
- ↑ 7.0 7.1 Schonegge, Anne-Marie, et al. "Evolutionary action and structural basis of the allosteric switch controlling β2AR functional selectivity." Nature, Nature Publishing Group, 18 December 2017, https://www.nature.com/articles/s41467-017-02257-x
- ↑ Sandoval, A., et al. "The Molecular Switching Mechanism at the Conserved D(E)RY Motif in Class-A GPCRs." Biophysical journal, 111(1), 79-89. https://doi.org/10.1016/j.bpj.2016.06.004
- ↑ Katritch V, Fenalti G, Abola EE, Roth BL, Cherezov V, Stevens RC. Allosteric sodium in class A GPCR signaling. Trends Biochem Sci. 2014 May;39(5):233-44. doi: 10.1016/j.tibs.2014.03.002. Epub , 2014 Apr 21. PMID:24767681 doi:http://dx.doi.org/10.1016/j.tibs.2014.03.002
- ↑ Babina, M., et al. "MRGPRX2 Is the Codeine Receptor of Human Skin Mast Cells: Desensitization through β-Arrestin and Lack of Correlation with the FcεRI Pathway." Journal of Investigative Dermatology, 141(6), 1286-1296. https://doi.org/10.1016/j.jid.2020.09.017
- ↑ McNeil, B. D., et al. "MRGPRX2 and Adverse Drug Reactions." Frontier Immunology, 06 August 2021, https://www.frontiersin.org/articles/10.3389/fimmu.2021.676354/full
- ↑ Ogasawara, H., et al. "Novel MRGPRX2 antagonists inhibit IgE-independent activation of human umbilical cord blood-derived mast cells." Journal of Leukocyte Biology, 12 July 2019, https://jlb.onlinelibrary.wiley.com/doi/10.1002/JLB.2AB1018-405R