Transcription-repair coupling factor
From Proteopedia
| (3 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| + | __TOC__ | ||
The bacterial [[transcription-repair coupling factor]] TRCF, also called Mfd translocase, is a DNA repair protein. It has a role in transcription-coupled repair, a subpathway of nucleotide excision repair (NER). Mfd recognizes stalled RNA polymerase (RNAP) and either restarts transcription or removes the stalled polymerase and recruits the NER proteins UvrA and UvrB. | The bacterial [[transcription-repair coupling factor]] TRCF, also called Mfd translocase, is a DNA repair protein. It has a role in transcription-coupled repair, a subpathway of nucleotide excision repair (NER). Mfd recognizes stalled RNA polymerase (RNAP) and either restarts transcription or removes the stalled polymerase and recruits the NER proteins UvrA and UvrB. | ||
| Line 24: | Line 25: | ||
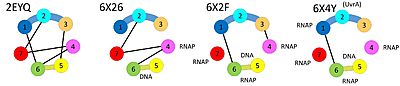
In the apo-structure, domains 3 and 4 are far apart from each other (connected by an extended linker), but they make direct contacts when Mfd is loaded on DNA. On the other hand, domains 4 and 5 make direct contacts in the apo-structure, but are far apart from each other (coonected by an extended linker) when Mfd is loaded on DNA. Finally, domain 2 and domain 7 bind to each other in the apo-state whereas domain 2 is available for binding UvrA in the DNA-bound state. | In the apo-structure, domains 3 and 4 are far apart from each other (connected by an extended linker), but they make direct contacts when Mfd is loaded on DNA. On the other hand, domains 4 and 5 make direct contacts in the apo-structure, but are far apart from each other (coonected by an extended linker) when Mfd is loaded on DNA. Finally, domain 2 and domain 7 bind to each other in the apo-state whereas domain 2 is available for binding UvrA in the DNA-bound state. | ||
| + | |||
== 3D Structures of Transcription-repair coupling factor == | == 3D Structures of Transcription-repair coupling factor == | ||
[[Transcription-repair coupling factor 3D structures]] | [[Transcription-repair coupling factor 3D structures]] | ||
| - | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
| - | |||
| - | == 3D Structures of Transcription-repair coupling factor == | ||
| - | |||
| - | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | |||
| - | [[2eyq]] – EcTRCF – ''Escherichia coli''<br /> | ||
| - | [[6x2n]], [[6x2f]], [[6x26]], [[6x50]], [[6x43]], [[6x4w]], [[6x4y]] - EcTRCF, RNA polymerase, RNA, DNA (Cryo EM)<br /> | ||
| - | [[3hjh]] – EcTRCF residues 1-470<br /> | ||
| - | [[2b2n]] - EcTRCF residues 1-333<br /> | ||
| - | [[6yhz]] - EcTRCF residues 472-547 – NMR<br /> | ||
| - | [[4dfc]] – EcTRCF D2 domain 127-213 + UvrABC system protein A<br /> | ||
| - | [[6xeo]] – EcTRCF + DNA – Cryo EM<br /> | ||
| - | [[6x26]] – EcTRCF in RNA polymerase complex – Cryo EM<br /> | ||
| - | [[6x2f]], [[6x4w]], [[6x4y]] – EcTRCF in RNA polymerase complex + ADP – Cryo EM<br /> | ||
| - | [[6x2n]], [[6x43]], [[6x50]] – EcTRCF in RNA polymerase complex + ATP – Cryo EM<br /> | ||
| - | [[3mlq]] – TtTRCF RNA polymerase interacting domain + DNA-directed RNA polymerase subunit β - ''Thermus thermophilus''<br /> | ||
| - | [[6m6a]] – TtTRCF in RNA polymerase complex – Cryo EM<br /> | ||
| - | [[6m6b]] – TtTRCF in RNA polymerase complex + ATP-γ-S – Cryo EM<br /> | ||
| - | [[2qsr]] – TRCF C terminal – ''Streptococcus pneumoni''<br /> | ||
| - | [[6ac6]], [[6aca]], [[6ac8]] – MsTRCF – ''Mycobacterium smegmatis''<br /> | ||
| - | [[6acx]] – MsTRCF + ADP<br /> | ||
| - | |||
[[Category: Escherichia coli]] | [[Category: Escherichia coli]] | ||
| Line 69: | Line 48: | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
<br /> | <br /> | ||
| + | </StructureSection> | ||
Created with the participation of [[User:Wayne Decatur|Wayne Decatur]] | Created with the participation of [[User:Wayne Decatur|Wayne Decatur]] | ||
Current revision
Contents |
The bacterial transcription-repair coupling factor TRCF, also called Mfd translocase, is a DNA repair protein. It has a role in transcription-coupled repair, a subpathway of nucleotide excision repair (NER). Mfd recognizes stalled RNA polymerase (RNAP) and either restarts transcription or removes the stalled polymerase and recruits the NER proteins UvrA and UvrB.
Function
Mfd has ATP hydrolysis activity, DNA binding sites and a UvrA binding sites. These three functions are inhibited in the isolated enzyme, but are activated when Mfd encounters stalled RNA polymerase (or through various mutations that remove inhibitory domains [1]). Mfd also contains an RNA interaction domain (RID) that binds to the beta subunit of RNAP.
Relationship to other enzymes
The N-terminal part of Mfd shows sequence similarity to UvrB, including in the domain of UvrB that interacts with UvrA. However, the conserved helicase motifs present in UvrB (responsible for binding and hydrolyzing ATP) are absent in that part of Mfd. Furthermore, the sequence segment known to fold as a beta hairpin in UvrB (involved in clamping down a single strand of DNA) seems absent. The C-terminal part of Mfd shows sequence similarity to SF1/SF2 helicases (UvrB is an example), containing conserved helicase/translocase motifs.
Structure and conformational change
| |||||||||||
Created with the participation of Wayne Decatur
Proteopedia Page Contributors and Editors (what is this?)
Karsten Theis, Michal Harel, Alexander Berchansky, Wayne Decatur