Sphingosine kinase
From Proteopedia
(Difference between revisions)
| (9 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <StructureSection load='3vzb' size='350' side='right' caption=' | + | <StructureSection load='3vzb' size='350' side='right' caption='Human sphingosine kinase, Type 1 complex with sphingosine, ethylene glycol and sulphate (PDB entry [[3vzb]])' scene=''> |
| + | __TOC__ | ||
==Structure Highlights== | ==Structure Highlights== | ||
<table><tr><td colspan='2'> For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=3VZB FirstGlance]. <br> | <table><tr><td colspan='2'> For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=3VZB FirstGlance]. <br> | ||
| Line 6: | Line 7: | ||
<tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">SPHK1, SPHK, SPK ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 HUMAN])</td></tr> | <tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">SPHK1, SPHK, SPK ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 HUMAN])</td></tr> | ||
<tr id='activity'><td class="sblockLbl"><b>Activity:</b></td><td class="sblockDat"><span class='plainlinks'>[http://en.wikipedia.org/wiki/Sphinganine_kinase Sphinganine kinase], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=2.7.1.91 2.7.1.91] </span></td></tr> | <tr id='activity'><td class="sblockLbl"><b>Activity:</b></td><td class="sblockDat"><span class='plainlinks'>[http://en.wikipedia.org/wiki/Sphinganine_kinase Sphinganine kinase], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=2.7.1.91 2.7.1.91] </span></td></tr> | ||
| - | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=3vzb FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3vzb OCA], [http://www.rcsb.org/pdb/explore.do?structureId=3vzb RCSB | + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=3vzb FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3vzb OCA], [http://www.rcsb.org/pdb/explore.do?structureId=3vzb RCSB]</span></td></tr> |
| + | |||
</table> | </table> | ||
| Line 18: | Line 20: | ||
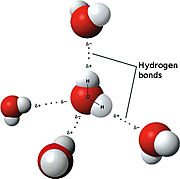
The <scene name='91/910628/Sphk1_surface/4'>catalytic site</scene> (used in converting sphingosine to S1P-see below in "Sphingolipids" section for more details) is in a <scene name='91/910628/Sphk1_hydrophobic_pocket/1'>'''hydrophobic''' cleft</scene>, located between the two domains. <ref name="wang">PMID:23602659</ref> This hydrophobic cleft is an '''ideal binding pocket''' for a '''hydrophobic lipid''', like sphingosine (an '''18-carbon''' amino alcohol with an '''unsaturated''' '''hydrocarbon''' chain). <ref name="wang">PMID:23602659</ref> The binding of sphingosine to the SphK1 hydrophobic "pocket" is mediated by both the anchoring of the <scene name='91/910628/Sphingosine_element_label/2'>hydrophilic (polar) headgroup</scene> of sphingosine to the protein surface and the presence of the hydrophobic alkyl chain in the interior of the protein. <ref name="wang">PMID:23602659</ref> The 2-amino-1,3-diol moiety of the sphingosine head group is situated at the cleft between the two domains, allowing for '''hydrogen-bond interactions''' ('''Figure 1''') with <scene name='91/910628/Amino_acid_interactions/4'>Asp81 through the 1-hydroxyl and with Asp178 through the 3-hydroxyl</scene>, which also forms a water-mediated hydrogen bond with Ser168. Sphingosine's long acyl chain is buried in the hydrophobic pocket ("tunnel shaped"), lined by side chains of mostly <scene name='91/910628/Amino_acid_interactions/6'>non-polar amino acid residues</scene> (i.e., Phe173, Ile174, Val177, Phe192, Thr196, Leu299, Leu302, Phe303, Met306, His311, Leu319, Phe288, Val290, Leu259, Leu261, Ala170, Met272, and Ala274). <ref name="wang">PMID:23602659</ref> | The <scene name='91/910628/Sphk1_surface/4'>catalytic site</scene> (used in converting sphingosine to S1P-see below in "Sphingolipids" section for more details) is in a <scene name='91/910628/Sphk1_hydrophobic_pocket/1'>'''hydrophobic''' cleft</scene>, located between the two domains. <ref name="wang">PMID:23602659</ref> This hydrophobic cleft is an '''ideal binding pocket''' for a '''hydrophobic lipid''', like sphingosine (an '''18-carbon''' amino alcohol with an '''unsaturated''' '''hydrocarbon''' chain). <ref name="wang">PMID:23602659</ref> The binding of sphingosine to the SphK1 hydrophobic "pocket" is mediated by both the anchoring of the <scene name='91/910628/Sphingosine_element_label/2'>hydrophilic (polar) headgroup</scene> of sphingosine to the protein surface and the presence of the hydrophobic alkyl chain in the interior of the protein. <ref name="wang">PMID:23602659</ref> The 2-amino-1,3-diol moiety of the sphingosine head group is situated at the cleft between the two domains, allowing for '''hydrogen-bond interactions''' ('''Figure 1''') with <scene name='91/910628/Amino_acid_interactions/4'>Asp81 through the 1-hydroxyl and with Asp178 through the 3-hydroxyl</scene>, which also forms a water-mediated hydrogen bond with Ser168. Sphingosine's long acyl chain is buried in the hydrophobic pocket ("tunnel shaped"), lined by side chains of mostly <scene name='91/910628/Amino_acid_interactions/6'>non-polar amino acid residues</scene> (i.e., Phe173, Ile174, Val177, Phe192, Thr196, Leu299, Leu302, Phe303, Met306, His311, Leu319, Phe288, Val290, Leu259, Leu261, Ala170, Met272, and Ala274). <ref name="wang">PMID:23602659</ref> | ||
| - | </StructureSection> | ||
[[Image:SphK1 Sequence and Structure Conservation Small.jpeg|300px|right|thumb|'''Figure 2'''. SphK1 Sequence Conservation Structure with Conservation Table in Figure 4]] | [[Image:SphK1 Sequence and Structure Conservation Small.jpeg|300px|right|thumb|'''Figure 2'''. SphK1 Sequence Conservation Structure with Conservation Table in Figure 4]] | ||
| Line 26: | Line 27: | ||
[https://en.wikipedia.org/wiki/Kinase '''Sphingosine Kinases'''] are part of distinct class of lipid kinases that is evolutionarily highly conserved ('''Figure 2 and 3''').<ref name="Kohama">PMID:9726979</ref> They do not share sequence homology with other lipid kinases, such as phosphotidylinositol-3 kinase [https://en.wikipedia.org/wiki/Phosphoinositide_3-kinase (PI3K)]('''Figure 3'''). <ref name="wang">PMID:23602659</ref> However, protein sequence motif analysis and structure classification suggested that SphKs belong to the phosphofructokinase [https://en.wikipedia.org/wiki/Phosphofructokinase (PFK)]-like superfamily <ref name="cheek">PMID:15771780</ref> <ref name="labesse">PMID:12069781</ref> sharing the same protein fold (i.e. the [[Rossmann fold]]) with NAD kinases, ceramide kinases, and phosphofructokinases. <ref name="wang">PMID:23602659</ref> | [https://en.wikipedia.org/wiki/Kinase '''Sphingosine Kinases'''] are part of distinct class of lipid kinases that is evolutionarily highly conserved ('''Figure 2 and 3''').<ref name="Kohama">PMID:9726979</ref> They do not share sequence homology with other lipid kinases, such as phosphotidylinositol-3 kinase [https://en.wikipedia.org/wiki/Phosphoinositide_3-kinase (PI3K)]('''Figure 3'''). <ref name="wang">PMID:23602659</ref> However, protein sequence motif analysis and structure classification suggested that SphKs belong to the phosphofructokinase [https://en.wikipedia.org/wiki/Phosphofructokinase (PFK)]-like superfamily <ref name="cheek">PMID:15771780</ref> <ref name="labesse">PMID:12069781</ref> sharing the same protein fold (i.e. the [[Rossmann fold]]) with NAD kinases, ceramide kinases, and phosphofructokinases. <ref name="wang">PMID:23602659</ref> | ||
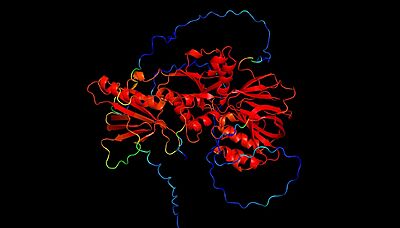
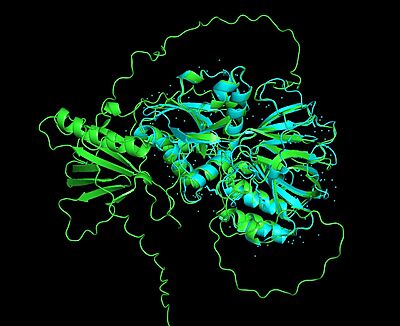
| - | The mammalian isoforms of sphingosine kinase, SphK1 and SphK2, are the only isoforms that have been characterized ('''Figure 4 and 5'''). Additionally, the crystal structure has been solved for SphK1 (via [[X-ray diffraction]] resulting in a resolution of 2.00Å) with SphK2 having a highly predictive [https://en.wikipedia.org/wiki/AlphaFold Alpha Fold] structure ('''Figure 4 and 5'''). <ref name="wang">PMID:23602659</ref> '''Figure 5''' shows the alignment of the crystal structure of SphK1 and the Alpha Fold prediction model of SphK2. Here, the catalytic domain aligns with high confidence (showing an RMSD value of 0. | + | The mammalian isoforms of sphingosine kinase, SphK1 and SphK2, are the only isoforms that have been characterized ('''Figure 4 and 5'''). Additionally, the crystal structure has been solved for SphK1 (via [[X-ray diffraction]] resulting in a resolution of 2.00Å) with SphK2 having a highly predictive [https://en.wikipedia.org/wiki/AlphaFold Alpha Fold] structure ('''Figure 4 and 5'''). <ref name="wang">PMID:23602659</ref> '''Figure 5''' shows the alignment of the crystal structure of SphK1 and the Alpha Fold prediction model of SphK2. Here, the catalytic domain aligns with high confidence (showing an RMSD value of 0.0846Å. However, the Alpha Fold prediction model of Sphk2 shows an entire domain (of α/β structure architecture) that does not align with the SphK1 crystal structure. As you will read further in the "Sphingosine Kinase (SphK1 and SphK2)" section, although SphK1 and SphK2 share similar structural properties and mechanistic properties, their kinetic regulation is different (a reason as to why there may be an additional domain predicted in the SphK2 structure). |
[[Image:SphK2 AlphaFold B-factor.jpeg|400px|left|thumb|'''Figure 4'''. SphK2 Alpha Fold Prediction Model, Labeled by B-factor (red=high confidence prediction, blue=low confidence prediction]] | [[Image:SphK2 AlphaFold B-factor.jpeg|400px|left|thumb|'''Figure 4'''. SphK2 Alpha Fold Prediction Model, Labeled by B-factor (red=high confidence prediction, blue=low confidence prediction]] | ||
| - | [[Image:SphK1 and SphK2 alignment Large.jpeg|400px|center|thumb|'''Figure 5'''. SphK1 and SphK2 Alignment, RMSD= 0. | + | [[Image:SphK1 and SphK2 alignment Large.jpeg|400px|center|thumb|'''Figure 5'''. SphK1 and SphK2 Alignment, RMSD= 0.846Å, SphK1=cyan, SphK2=lime green]] |
== Sphingolipids == | == Sphingolipids == | ||
| Line 44: | Line 45: | ||
== Importance of SphKs in Pathogen Defense and Immune Response == | == Importance of SphKs in Pathogen Defense and Immune Response == | ||
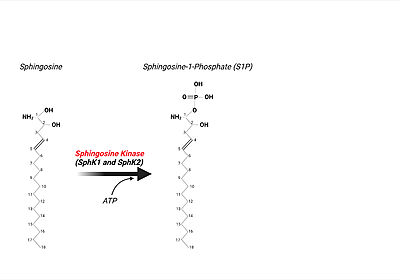
Upon the '''SphK ATP-dependent phosphorylation event of sphingosine to S1P''', the phosphorylated lipid product can be utilized in many intracellular and extracellular signaling mechanisms. <ref name="Drexler">PMID:15289826</ref> One '''critical''' function of S1P (through [https://en.wikipedia.org/wiki/Autocrine_signaling autocrine] or [https://en.wikipedia.org/wiki/Paracrine_signaling paracrine] extracellular signaling), is eliciting the immune response when pathogens are present or causing infection. <ref name="Bryan">PMID:15289826</ref> In order to "kick-start" the immune response, first, S1P is transported out of the cell by Spinster Homologue 2, SPNS2, a member of a large family of non ATP-dependent [https://en.wikipedia.org/wiki/Ion_transporter ion transporters]. <ref name="Spiegal2">PMID:30655317</ref> After the lipid's transport out of eukaryotic cell, S1P can bind to one of '''5 S1P G-protein coupled receptors (GPCRs)''' present on the surface of both innate and adaptive immune cell types resulting in immune cell trafficking to the site of infection. <ref name="Spiegal2">PMID:30655317</ref> <ref name="Bryan">PMID:15289826</ref> For example, [https://en.wikipedia.org/wiki/Macrophage macrophages] express S1P receptors I, II, III, and IV. Upon binding of S1P to S1P receptor type I, macrophages are signaled to be trafficked and recruited to the site of pathogen infection. <ref name="Bryan">PMID:15289826</ref> | Upon the '''SphK ATP-dependent phosphorylation event of sphingosine to S1P''', the phosphorylated lipid product can be utilized in many intracellular and extracellular signaling mechanisms. <ref name="Drexler">PMID:15289826</ref> One '''critical''' function of S1P (through [https://en.wikipedia.org/wiki/Autocrine_signaling autocrine] or [https://en.wikipedia.org/wiki/Paracrine_signaling paracrine] extracellular signaling), is eliciting the immune response when pathogens are present or causing infection. <ref name="Bryan">PMID:15289826</ref> In order to "kick-start" the immune response, first, S1P is transported out of the cell by Spinster Homologue 2, SPNS2, a member of a large family of non ATP-dependent [https://en.wikipedia.org/wiki/Ion_transporter ion transporters]. <ref name="Spiegal2">PMID:30655317</ref> After the lipid's transport out of eukaryotic cell, S1P can bind to one of '''5 S1P G-protein coupled receptors (GPCRs)''' present on the surface of both innate and adaptive immune cell types resulting in immune cell trafficking to the site of infection. <ref name="Spiegal2">PMID:30655317</ref> <ref name="Bryan">PMID:15289826</ref> For example, [https://en.wikipedia.org/wiki/Macrophage macrophages] express S1P receptors I, II, III, and IV. Upon binding of S1P to S1P receptor type I, macrophages are signaled to be trafficked and recruited to the site of pathogen infection. <ref name="Bryan">PMID:15289826</ref> | ||
| + | ==3D structures of sphingosine kinase== | ||
| + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| + | [[4v24]] – hSphK1 kinase domain 1-363 + pyrrolidine derivative – human<br /> | ||
| + | [[3vzb]] – hSphK1 kinase domain + sphingosine <br /> | ||
| + | [[3vzc]], [[4l02]] – hSphK1 kinase domain + inhibitor <br /> | ||
| + | [[3vzd]] – hSphK1 kinase domain + inhibitor + ADP<br /> | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | </StructureSection> | ||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||