We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
GLUT1
From Proteopedia
(Difference between revisions)
| (7 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
==Facilitated Glucose Transporter 1, Solute Carrier Family 2, ''Homo sapiens''== | ==Facilitated Glucose Transporter 1, Solute Carrier Family 2, ''Homo sapiens''== | ||
| - | <StructureSection load='4pyp' size='340' side='right' caption=' | + | <StructureSection load='4pyp' size='340' side='right' caption='Glucose ABC transporter complex with nonyl beta-D-glucopyranoside (PDB code [[4pyp]])' scene=''> |
| + | __TOC__ | ||
== Classification == | == Classification == | ||
| - | GLUT proteins, encoded by the SLC2 genes, are part of the Major Facilitator Superfamily (MFS) of substrate transporters. Structural elements characteristic of GLUT proteins are twelve transmembrane domains, one N-linked glycosylation site, and lengths of about five-hundred amino acids.<ref>PMID:23506862</ref> GLUT proteins transport a variety of monosaccharides that enable cellular respiration and are thus abundant in the body. Referencing the [http://cathdb.info/version/v4_3_0/superfamily/1.20.1250.20 CATH] classification database, the MFS superfamily has 23,982 unique species and 134 characteristic domains. GLUT1 has two highly-conserved ATP-binding domains known as Walker motifs A and B.<ref>PMID:15326030</ref> A third ATP-binding domain is less conserved. According to [https://www.uniprot.org/uniprot/?query=job:P2022050392C7BAECDB1C5C413EE0E0348724B682005B3CL&columns=id,entry%20name,reviewed,protein%20names,genes,organism,length,peptidesearch(P2022050392C7BAECDB1C5C413EE0E0348724B682005B3CL) UniProt], these specific sequences in the GLUT1 transporter are shared among glucose transporters in several different organisms. | + | GLUT proteins, encoded by the [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4104978/ SLC2] genes, are part of the Major Facilitator Superfamily (MFS) of substrate transporters. Structural elements characteristic of GLUT proteins are twelve transmembrane domains, one N-linked glycosylation site, and lengths of about five-hundred amino acids.<ref>PMID:23506862</ref> GLUT proteins transport a variety of monosaccharides that enable cellular respiration and are thus abundant in the body. Referencing the [http://cathdb.info/version/v4_3_0/superfamily/1.20.1250.20 CATH] classification database, the MFS superfamily has 23,982 unique species and 134 characteristic domains. GLUT1 has two highly-conserved ATP-binding domains known as Walker motifs A and B.<ref>PMID:15326030</ref> A third ATP-binding domain is less conserved. According to [https://www.uniprot.org/uniprot/?query=job:P2022050392C7BAECDB1C5C413EE0E0348724B682005B3CL&columns=id,entry%20name,reviewed,protein%20names,genes,organism,length,peptidesearch(P2022050392C7BAECDB1C5C413EE0E0348724B682005B3CL) UniProt], these specific sequences in the GLUT1 transporter are shared among glucose transporters in several different organisms. |
| - | + | ||
| - | + | ||
== Function == | == Function == | ||
| Line 51: | Line 50: | ||
<scene name='91/910668/Glut1_default_view_charged/1'>GLUT1</scene> has a <scene name='91/910668/Glut1_hydrophobic_pocket/1'>hydrophobic pocket</scene> and is proposed to be comprised of <scene name='91/910668/Glut1_hps_2/1'>six amino acids</scene><scene name='91/910668/Glut1_hps_2/5'>(view 2)</scene>. In the structure [[4pyp]], these residues are Gly27, Thr30, Ile164, Val165, Ile168, and Phe291. This hydrophobic pocket has been proposed to facilitate substrate binding and unbinding between the "occluded" and "inward-open" conformations.<ref>PMID:27128978</ref> In this crystal structure, <scene name='91/910668/B-ng_in_4pyp/1'>N-nonyl-β-D-glucopyranoside (β-NG)</scene> acts as a glucose analog that binds the hydrophobic pocket.<ref>PMID:24847886</ref> | <scene name='91/910668/Glut1_default_view_charged/1'>GLUT1</scene> has a <scene name='91/910668/Glut1_hydrophobic_pocket/1'>hydrophobic pocket</scene> and is proposed to be comprised of <scene name='91/910668/Glut1_hps_2/1'>six amino acids</scene><scene name='91/910668/Glut1_hps_2/5'>(view 2)</scene>. In the structure [[4pyp]], these residues are Gly27, Thr30, Ile164, Val165, Ile168, and Phe291. This hydrophobic pocket has been proposed to facilitate substrate binding and unbinding between the "occluded" and "inward-open" conformations.<ref>PMID:27128978</ref> In this crystal structure, <scene name='91/910668/B-ng_in_4pyp/1'>N-nonyl-β-D-glucopyranoside (β-NG)</scene> acts as a glucose analog that binds the hydrophobic pocket.<ref>PMID:24847886</ref> | ||
| - | The GLUT1 transporter also has three | + | The GLUT1 transporter also has three ATP-binding sites. The lone <scene name='91/910668/Glut1_atp_binding_1/3'>extracellular ATP-binding site</scene> is proposed to be comprised of the residues Gly111, Phe112, Ser113, Lys114, Leu115, Gly116, Lys117, and Ser118. This is a domain consistent with Walker Motif A (G-X-X-G/X-X-G-K-T/X). The <scene name='91/910668/Glut1_atp_binding_2/3'>second ATP-binding site</scene> is one of two in the cytoplasmic portion of the protein. The residues comprising this ATP-binding site are Lys225, Ser226, Val227, Leu228, and Lys229. The <scene name='91/910668/Atp_binding_site_3/5'>third ATP-binding site</scene>, also localized to the cytoplasm, is comprised of the amino acids Gly332, Arg 333, Arg334, Thr335, Leu336, His337, and Leu338. This sequence is consistent with Walker Motif B (G-X-X-X-L-X-X).<ref>PMID:11425315</ref> As mentioned earlier in this page, both Walker motifs A and B are highly conserved. Some studies on GLUT1 show that ATP binding to the cytosolic domains causes C-terminus binding to the C-terminal side of the intracellular loop of the protein, preventing substrate import. ATP binding is not known to have any effects when binding extracellularly.<ref>PMID:25715702</ref> |
Several types of GLUT1 inhibitors exist, one being [https://en.wikipedia.org/wiki/Cytochalasin_B cytochalasin b]. Two Trp residues, Trp388 and Trp412, are thought to play a major role in <scene name='91/910668/Glut1_cytochalasin_b_1/1'>cytochalasin b binding to GLUT1</scene> via hydrophobic interactions.<ref>PMID:7078104</ref> | Several types of GLUT1 inhibitors exist, one being [https://en.wikipedia.org/wiki/Cytochalasin_B cytochalasin b]. Two Trp residues, Trp388 and Trp412, are thought to play a major role in <scene name='91/910668/Glut1_cytochalasin_b_1/1'>cytochalasin b binding to GLUT1</scene> via hydrophobic interactions.<ref>PMID:7078104</ref> | ||
There is at least one known amino acid substitution in GLUT1 that can cause GLUT1 deficiency syndrome. <scene name='91/910668/Glut1_arg126_1/1'>Arg126</scene> causes transmembrane helix #4 to become kinked, blocking substrate transport. Arg126 is the amino acid most often mutated in GLUT1 deficiency syndrome.<ref>PMID:18387950</ref> | There is at least one known amino acid substitution in GLUT1 that can cause GLUT1 deficiency syndrome. <scene name='91/910668/Glut1_arg126_1/1'>Arg126</scene> causes transmembrane helix #4 to become kinked, blocking substrate transport. Arg126 is the amino acid most often mutated in GLUT1 deficiency syndrome.<ref>PMID:18387950</ref> | ||
| + | |||
| + | GLUT1, and other glucose transporters, have an <scene name='91/910668/Ich_domain_1/1'>intracellular helices (ICH) domain</scene>. The ICH domain of GLUT1 may play a role in stabilizing the outward-facing conformation of the enzyme based on computer modeling in [https://en.wikipedia.org/wiki/MODELLER MODELLER].<ref>PMID:25919356</ref> Two residues of interest in the ICH domain are Arg212 and Asp240. These residues are hypothesized to participate in hydrogen bonding between the ICH domain and transmembrane domains of the protein with the cumulative effect of stabilizing the outward-facing conformation. | ||
| + | |||
| + | == Summary == | ||
| + | |||
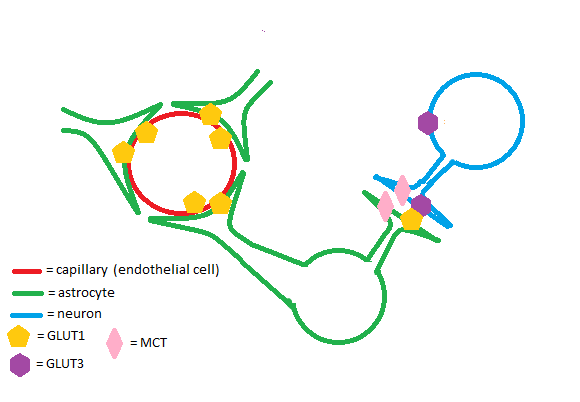
| + | GLUT1 is a glucose transporter expressed throughout the body. Increased expression of the protein is noted in tissues such as blood-brain barrier, the placenta, and the retina. GLUT1 works in conjunction with other solute carrier proteins to ensure that metabolically-demanding tissues receive a steady supply of substrates for ATP production. GLUT1 is implicated in a variety of pathologies such as GLUT1 deficiency syndrome, cancer, diabetes, and more. Dozens of studies on GLUT1 structure have revealed domains important in glucose binding and possibly allosteric regulation. Further research is necessary to fully understand GLUT1 function, but progress in biochemistry has provided many clues as to how GLUT1 works. | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
Facilitated Glucose Transporter 1, Solute Carrier Family 2, Homo sapiens
| |||||||||||