User:Clara Costa D'Elia/Sandbox 1
From Proteopedia
| (11 intermediate revisions not shown.) | |||
| Line 2: | Line 2: | ||
<Structure load='1KZU' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | <Structure load='1KZU' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | ||
| - | |||
| - | This is a default text for your page '''Clara Costa D'Elia/Sandbox 1'''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | ||
| - | You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | ||
==introduction== | ==introduction== | ||
Photosynthesis is the main source of energy used by life on earth nowadays. "The beginning of photosynthesis starts with the absorption of sunlight by an arrangement of photosynthetic pigments embedded into a proteic matrix called the light harvesting (LH) antenna complexes. The excitation energy of | Photosynthesis is the main source of energy used by life on earth nowadays. "The beginning of photosynthesis starts with the absorption of sunlight by an arrangement of photosynthetic pigments embedded into a proteic matrix called the light harvesting (LH) antenna complexes. The excitation energy of | ||
| - | photosynthetic pigments is then transferred to the photosynthetic reaction center where it is converted into chemical energy" | + | photosynthetic pigments is then transferred to the photosynthetic reaction center where it is converted into chemical energy".<ref name="M. Belén Oviedo and Cristián G. Sánchez">M. Belén Oviedo and Cristián G. Sánchez The Journal of Physical Chemistry A 2011 115 (44), 12280-12285 doi: //doi.org/10.1021/jp203826q </ref> Photosynthesis is divided in a few steps in a way that the antenna system does not do any chemistry, since it only works by transfering energy in the state of excited electrons between molecules. According to <ref name="Blankenship, R">Blankenship, R. E. Molecular Mechanisms of Photosynthesis; |
| - | .<ref> | + | |
Blackwell Science Ltd.: Oxford, U.K., 2002.</ref> this is only possible due to a weak energetic coupling | Blackwell Science Ltd.: Oxford, U.K., 2002.</ref> this is only possible due to a weak energetic coupling | ||
of the antenna pigments, which are bound to proteins in highly specific associations. | of the antenna pigments, which are bound to proteins in highly specific associations. | ||
| Line 15: | Line 11: | ||
Is it important to notice that this page describes the LHC II of purple bacterias, more specifically the one found in Rhodopseudomonas acidophila, which caries a similiar name but has nothing to do to the LHC II of plants and algae. | Is it important to notice that this page describes the LHC II of purple bacterias, more specifically the one found in Rhodopseudomonas acidophila, which caries a similiar name but has nothing to do to the LHC II of plants and algae. | ||
| - | IN general Purple bacteria contain two types of antenna complexes, integral membrane proteins, the light harvesting complex I and II. LHI associates with the reaction center and is always present, constituting part of the "core complex" as explained by <ref>https://doi.org/10.1038/374517a0</ref>. | + | IN general Purple bacteria contain two types of antenna complexes, integral membrane proteins, the light harvesting complex I and II. LHI associates with the reaction center and is always present, constituting part of the "core complex" as explained by <ref name="MCDERMOTT, G. M. et al">MCDERMOTT, G. M. et al. Crystal structure of an integral membrane light-harvesting complex from photosynthetic bacteria. Nature, v. 374, n. 6522, p. 517-521, 1995.https://doi.org/10.1038/374517a0</ref> |
| - | Light harvest complex | + | . |
| + | Light harvest complex two, also called LHII is located on the periphery of this core complex and its not always present, since its produced by the bacteria as an acessory complex depending on the availability of light levels encountered by the organism, in order to increase its range of absorption(Zuber & Brunisholz, 1991). Its important to notice that Both types of complex are built on a similar modular principle. | ||
| - | When purple bacteria are grown under anaerobic conditions they incorporate the photosynthetic apparatus described above into invaginated intracytoplasmic phospholipid membranes.<ref | + | When purple bacteria are grown under anaerobic conditions they incorporate the photosynthetic apparatus described above into invaginated intracytoplasmic phospholipid membranes.<ref name="MCDERMOTT, G. M. et al"/> |
| Line 24: | Line 21: | ||
| - | As mentioned light-harvesting complexes are important for Purple bacterial to maximize the spectrum of light | + | As mentioned light-harvesting complexes are important for Purple bacterial to maximize the spectrum of light available to them, modifying the absorption properties of their chromophores; |
| - | In the LHC The proteins help to determine the disposition of the pigments, with, as it will be explained, end up influencing their absorption spectra, together with other factors such as inter-pigment geometries and their interactions with protein and membrane environments.<ref> | + | In the LHC The proteins help to determine the disposition of the pigments, with, as it will be explained, end up influencing their absorption spectra, together with other factors such as inter-pigment geometries and their interactions with protein and membrane environments.<ref name="PAPIZ, Miroslav Z. et al">PAPIZ, Miroslav Z. et al. The structure and thermal motion of the B800–850 LH2 complex from Rps. acidophila at 2.0 Å resolution and 100 K: new structural features and functionally relevant motions. Journal of molecular biology, v. 326, n. 5, p. 1523-1538, 2003.</ref> |
| - | Pigmentwise, purple bacterias in general contain bacteriochlorophyll a or b, never both and in the case of Rhodopseudomonas acidophila, bacteriochlorophyll a, also abreviated to BChl a, associated with carotenoids, non-covalently bound to two low-molecular-weight hydrophobic apoproteins <ref | + | Pigmentwise, purple bacterias in general contain bacteriochlorophyll a or b, never both and in the case of Rhodopseudomonas acidophila, bacteriochlorophyll a, also abreviated to BChl a, associated with carotenoids, non-covalently bound to two low-molecular-weight hydrophobic apoproteins <ref name="MCDERMOTT, G. M. et al"/> |
| - | <scene name='91/911263/Bcl_with_mg/1'>Bacteriochlorophyll a</scene>It is the principal chlorophyll-type pigment in the majority of anoxygenic photosynthetic bacteria and | + | <scene name='91/911263/Bcl_with_mg/1'>Bacteriochlorophyll a</scene>It is the principal chlorophyll-type pigment in the majority of anoxygenic photosynthetic bacteria and acomodate several structural changes in comparison to the typical clorophin pigments, specially regarded its symmetry, witch impose effects on its spectral properties. |
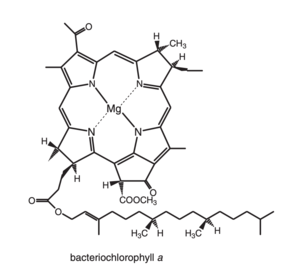
[[Image:Bacteriochlorophylla.png|300px|left|thumb|Chemical structure of Bacteriochlorophyll a. image from R. E. Molecular Mechanisms of Photosynthesis]] | [[Image:Bacteriochlorophylla.png|300px|left|thumb|Chemical structure of Bacteriochlorophyll a. image from R. E. Molecular Mechanisms of Photosynthesis]] | ||
| + | <ref name="Blankenship, R"/> | ||
| - | < | + | By clicking <scene name='91/911263/Bcl_with_mg/1'>Bacteriochlorophyll a</scene> is possible to visualize that there are two types of bacteriochlorophylll in different conformations regarding the whole complex, which explains why this pigments can absorb in two different frequencies. We can visualize there are B800 pigments which form a ring parallel to the plane of the membrane that the complex is embedded in. According to the same authors The B800 pigments "are weakly coupled to each other and are at a distance of approximately 21 Å from each other". This independence they keep as molecules answer for their spectral absorption range. |
| - | + | The rest of the bacteriochlorophylls can be seen arranged perpendicularly to the plane of the membrane and consist of The pigments that absorb at 850. Each subunit complex has two molecules of bacteriochlorophyll B850 arranged as a closely coupled dimer, which constitute a band of 16 or 18 pigments when the subunit complexes assemble into the LH2 complex. "This band of B850 pigments are all strongly exciton-coupled together, so excitations are effectively delocalized over much or all of the entire band, rather than being effectively localized in a single pigment for a short while and then hopping to another pigment, as is the case with the B800 pigments". | |
| - | By clicking <scene name='91/911263/Bcl_with_mg/1'>Bacteriochlorophyll a</scene> is possible to visualise that there are two types of bacteriochlorophylll in diferent conformations regarding the whole complex, which explains why this pigments can absorb in two diferent frequencys. We can visualisy there are B800 pigments wich form a ring parallel to the plane of the | ||
| - | membrane that the complex is embedded in. Acording to the same authors The B800 pigments "are weakly coupled to each other and are at a distance of approximately 21 Å from each other". This independence hey keep as molecules anwer for their spectral absorption range. | ||
| - | The rest of the bacteriochlorophylls can be seen arranged perpendicularly to the plane of the membrane and consist of The pigments that absorb at 850. Each subunit complex has two molecules of bacteriochlorophyll B850 arranged as a closely coupled dimer, which constitue a band of 16 or 18 pigments when the subunit complexes assemble into the LH2 complex. "This band of B850 pigments are all strongly exciton-coupled together, so excitations are effectively delocalized over much or all of the entire band, rather than being effectively localized in a single pigment for a short while and then hopping to another pigment, as is the case with the B800 pigments". | ||
| - | + | To further understand the difference on absorbance between the bacteriochlorophylls in a more independet state and in a more conjugated state is important to understand the concept of delocalization of energy, which is the extra stability a compound has as a result of having delocalized electrons.<ref name="Libretext">https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Chemical_Bonding/Valence_Bond_Theory/Delocalization_of_Electrons</ref> " Electron delocalization is also called resonance. Therefore, delocalization energy is also called resonance energy." | |
| - | To further understand the | + | |
"Charge delocalization is a stabilizing force because it spreads energy over a larger area rather than keeping it confined to a small area. Since electrons are charges, the presence of delocalized electrons brings extra stability to a system compared to a similar system where electrons are localized." | "Charge delocalization is a stabilizing force because it spreads energy over a larger area rather than keeping it confined to a small area. Since electrons are charges, the presence of delocalized electrons brings extra stability to a system compared to a similar system where electrons are localized." | ||
| - | Since conjugation brings up electron delocalization, it follows that the more extensive the conjugated system, like B850 molecules, the more stable | + | Since conjugation brings up electron delocalization, it follows that the more extensive the conjugated system, like B850 molecules, the more stable it is, at the same time that its potential energy is lower <ref name:<ref name="Libretext"/> |
| Line 53: | Line 47: | ||
| - | Acordig to <ref | + | Acordig to <ref name="Blankenship, R"/>, The spectra of photosynthethic pigments in general can be described by using the “four orbital” model. In this model there are four p molecular orbitals involved, simplified as HOMOs (highest occupied molecular orbitals) and LUMOs ( lowest unoccupied molecular orbitals). |
| - | + | ||
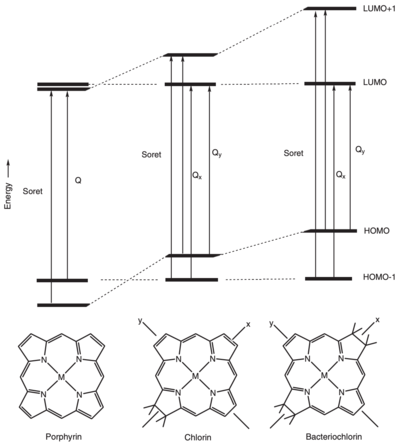
[[Image:Orbitais.png|400px|right|thumb|Molecular orbital energy level diagram of porphyrin, chlorin and bacteriochlorin.A very | [[Image:Orbitais.png|400px|right|thumb|Molecular orbital energy level diagram of porphyrin, chlorin and bacteriochlorin.A very | ||
simplified representation of the different electronic transitions is indicated. image from R. E. Molecular Mechanisms of Photosynthesis]] | simplified representation of the different electronic transitions is indicated. image from R. E. Molecular Mechanisms of Photosynthesis]] | ||
The two lowest-energy transitions are called the Q bands, they are in the visible region | The two lowest-energy transitions are called the Q bands, they are in the visible region | ||
| - | of the spectrum and are the most important for the photophysics, and the two higher-energy ones are known as the B bands or Soret bands, and are found in the UV region composing of a large series of electronic transitions.<ref | + | of the spectrum and are the most important for the photophysics, and the two higher-energy ones are known as the B bands or Soret bands, and are found in the UV region composing of a large series of electronic transitions.<ref name="M. Belén Oviedo and Cristián G. Sánchez"/> |
| - | <ref | + | <ref name="MCDERMOTT, G. M. et al"/>. |
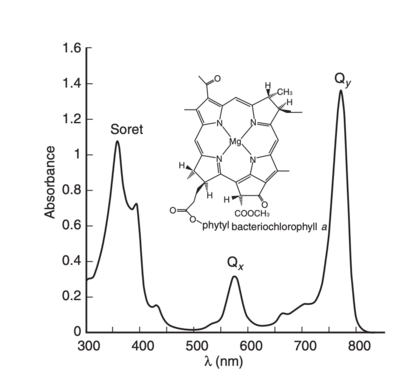
[[Image:Bacteriochlorophyllaabs.png|400px|left|thumb|Absorption spectra of bacteriochlorophyll a. image from R. E. Molecular Mechanisms of Photosynthesis]] | [[Image:Bacteriochlorophyllaabs.png|400px|left|thumb|Absorption spectra of bacteriochlorophyll a. image from R. E. Molecular Mechanisms of Photosynthesis]] | ||
| Line 68: | Line 61: | ||
| - | In order to further understand how the pigments respond to light excitation, its important to visually the | + | In order to further understand how the pigments respond to light excitation, its important to visually the molecules x and y axis. By convention, the y molecular axis is defined as the axis passing through the N atoms of rings A and C as show in the figure; |
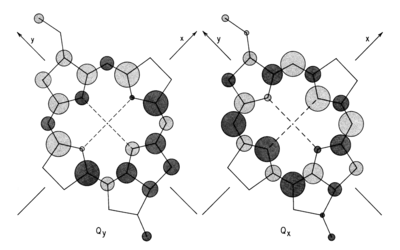
[[Image:Qy 2.png|300px|right|thumb|Chlorophyll a molecular model showing the main molecular axes and the transition dipole vector of the Qy band. Image from Oviedo, M. Belén, and Cristián G. Sánchez. "Transition Dipole Moments of the Q y Band in Photosynthetic Pigments." The Journal of Physical Chemistry A 115.44 (2011): 12280-12285.]] | [[Image:Qy 2.png|300px|right|thumb|Chlorophyll a molecular model showing the main molecular axes and the transition dipole vector of the Qy band. Image from Oviedo, M. Belén, and Cristián G. Sánchez. "Transition Dipole Moments of the Q y Band in Photosynthetic Pigments." The Journal of Physical Chemistry A 115.44 (2011): 12280-12285.]] | ||
| - | <ref | + | <ref name="M. Belén Oviedo and Cristián G. Sánchez"/>. Acording to <ref name="Blankenship, R"/> "The longest-wavelength transition is invariably polarized along the y axis of the molecule and is therefore known as the Qy transition. This means that the absorption will be strongest if the electric vector of plane-polarized exciting light is parallel to the y molecular axis of the pigment. The exciting light couples to the p electrons of the |
| - | + | ||
| - | axis of the molecule and is therefore known as the Qy transition. This means that the absorption will be strongest if the electric vector of plane-polarized exciting light is parallel to the y molecular axis of the pigment. The exciting light couples to the p electrons of the | + | |
molecule and rearranges them somewhat during the transition". | molecule and rearranges them somewhat during the transition". | ||
This Qy transition causes a shift in electron density along the axis and its called a transition dipole. | This Qy transition causes a shift in electron density along the axis and its called a transition dipole. | ||
| Line 90: | Line 81: | ||
==Energy transfer mechanism== | ==Energy transfer mechanism== | ||
| - | When a Bchl molecule is excited by light, its first excited | + | When a Bchl molecule is excited by light, its first excited state lasts for a few nanoseconds, therefore energy transfer within the LH2 complex can occur from the BSOO to the B850 BChl a molecules in just 0.7 ps, transfering the absorbed energy to the reaction centre. |
| - | + | Some of the important features that allow this to take place are revealed by its structure, such as the ultrafast depolarization that take place once the energy reaches the B850 molecules(on the 200-300 fs timescale).<ref name="MCDERMOTT, G. M. et al"/> | |
| - | + | ||
| - | <ref | + | |
| + | The ring of B850 Bchl a molecules acts like a 'storage ring', with the excited state rapidly delocalized over a large area, facilitated by a highly hydrophobic environment which reduces the dielectric constant, allowing coupling over large distances. The energy is then available for transfer from any part of the ring to any neighbouring LHI complex.<ref name="MCDERMOTT, G. M. et al"/> | ||
| + | There are many structural similarities between LHI and LHII, such as the connections between their bacteriochlorophyll to their histidine residues, guaranteeing that their bacteriochlorophyll rings are at the same orientation, which facilitate the energy transfer and create no requirement for the | ||
| + | LHI complex to have a special orientation to receive energy from the LH2 ring. | ||
| + | The facility of energy transfer between LHII and LHI lead to a further kinetic gains. | ||
| + | Studies also have shown that the carotenoid in this LH2 complex acts as an efficient accessory light-harvesting pigment (>50%)7. The excited singlet lifetime of carotenoids is usually less than 10 pS8. Therefore, if energy transfer is to compete successfully with these rapid de-excitation processes, the carotenoid must be located very close to the acceptor bacteriochlorophylls, as seen in the structure. | ||
| + | <ref name="MCDERMOTT, G. M. et al"/> | ||
| - | + | The carotenoid molecules have an extended conformation and lie generally perpendicular to the plane of the membrane, with close approach (3.4–3.7 Å) to both the B800 and the B850 pigments. | |
| - | The carotenoid molecules have an extended conformation and lie generally perpendicular to the plane of the membrane, with close approach (3.4–3.7 Å) to both the B800 | + | |
| - | and the B850 pigments. | + | |
==Structure== | ==Structure== | ||
| Line 106: | Line 99: | ||
<Structure load='1KZU' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | <Structure load='1KZU' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | ||
| - | The differences between the LH1 and LH2 complexes reside in their protein/pigment stoichiometry and modes of oligomerization. Structural studies have shown that LH2 complexes are formed from nine <scene name='91/911263/Helix_with_bcl/1'>αβ subunit oligomers</scene>, organized in a ring of inner a <scene name='91/911263/Inner_a_helix_with_bcl/2'>a-peptide</scene>(hollow cylinder of radius 18 A) and outer <scene name='91/911263/Outer_b_helix_with_bcl/1'>b-peptides</scene> (outer cylinder of radius 34 A). The a-apoprotein contains 53 amino acids, forming a hollow cylinder of radius 18 A and the b-apoprotein contains 41 amino acids and are arranged radially with the a-apoproteins to form an outer cylinder of radius 34 A. The a-apoprotein helices are parallel to the ninefold axis to within 2°, and the | + | The differences between the LH1 and LH2 complexes reside in their protein/pigment stoichiometry and modes of oligomerization. Structural studies have shown that LH2 complexes are formed from nine <scene name='91/911263/Helix_with_bcl/1'>αβ subunit oligomers</scene>, shown in puprle, organized in a ring of inner a <scene name='91/911263/Inner_a_helix_with_bcl/2'>a-peptide</scene>(hollow cylinder of radius 18 A) in light blue and outer <scene name='91/911263/Outer_b_helix_with_bcl/1'>b-peptides</scene> (outer cylinder of radius 34 A) in dark blue. The a-apoprotein contains 53 amino acids, forming a hollow cylinder of radius 18 A and the b-apoprotein contains 41 amino acids and are arranged radially with the a-apoproteins to form an outer cylinder of radius 34 A. The a-apoprotein helices are parallel to the ninefold axis to within 2°, and the b-apoprotein helices are inclined by 15° relative to this axis |
| - | <ref | + | <ref name="MCDERMOTT, G. M. et al"/> |
The Bchl a-binding histidines of the a (His 31) and b (His 30) apoproteins face outwards and inwards, respectively, forming a complete ring of 18 overlapping Bchl a molecules. For these molecules, the planes of the bacteriochlorins are parallel to | The Bchl a-binding histidines of the a (His 31) and b (His 30) apoproteins face outwards and inwards, respectively, forming a complete ring of 18 overlapping Bchl a molecules. For these molecules, the planes of the bacteriochlorins are parallel to | ||
| - | the membrane normal and their | + | the membrane normal and their centers are approximately 10A from the presumed periplasmic membrane surface. The nine |
| - | remaining Bchl a molecules are packed between the | + | remaining Bchl a molecules are packed between the b-apoprotein helices a further 16.5 A into the membrane with their bacteriochlorin rings parallel to the membrane surface. <ref name="Prince">PRINCE, S. M. et al. Apoprotein structure in the LH2 complex from Rhodopseudomonas acidophila strain 10050: modular assembly and protein pigment interactions. Journal of molecular biology, v. 268, n. 2, p. 412-423, 1997.</ref> |
''' The apoprotein subunits''' | ''' The apoprotein subunits''' | ||
| Line 118: | Line 111: | ||
| - | Proceeding from the N-terminal a-helice, a loop involved in the coordination and environ of a Bchl a molecule gives way to a short section of <scene name='91/911263/310_helice/5'>310 helice</scene>. The 310 helix penetrates the plane of the membrane surface and its linked to a seven-turn transmembrane a-helix, which then links to a two-turn amphipathic helix on periplasmic surface of the membrane. These small terminal helices might act as anchors to the transmembrane helixes perpendicular to the membrane surfaces. | + | Proceeding from the N-terminal a-helice, a loop involved in the coordination and environ of a Bchl a molecule gives way to a short section of <scene name='91/911263/310_helice/5'>310 helice</scene>. The 310 helix penetrates the plane of the membrane surface and its linked to a seven-turn transmembrane a-helix, which then links to a two-turn amphipathic helix on periplasmic surface of the membrane. These small terminal helices might act as anchors to the transmembrane helixes perpendicular to the membrane surfaces.<ref name="Prince"/> |
| - | The fold of the <scene name='91/911263/B_apoprotein/1'>b-apoprotein</scene> is similar, although shorter. Unexpectedly, the only direct interaction between the helices is at the N- and C-terminal ends. Within membrane-buried portions of the helices, interactions are mediated via the pigment molecules or buried water. | + | The fold of the <scene name='91/911263/B_apoprotein/1'>b-apoprotein</scene> is similar, although shorter. Unexpectedly, the only direct interaction between the helices is at the N- and C-terminal ends. Within membrane-buried portions of the helices, interactions are mediated via the pigment molecules or buried water.<ref name="Prince"/> |
| - | The eighteen B850 Bchl a molecules are supported by <scene name='91/911263/Histidine_residue/1'>histidine residues</scene> on the a- and b-apoproteins with their bacteriochlorin rings perpendicular to the membrane surface and associated phytyl chains descending into the hydrophobic core of the assembly. In contrast, the B800 Bchl a molecules have their bacteriochlorin rings aligned parallel to the membrane surface | + | The eighteen B850 Bchl a molecules are supported by <scene name='91/911263/Histidine_residue/1'>histidine residues</scene> on the a- and b-apoproteins with their bacteriochlorin rings perpendicular to the membrane surface and associated phytyl chains descending into the hydrophobic core of the assembly. In contrast, the B800 Bchl a molecules have their bacteriochlorin rings aligned parallel to the membrane surface. <ref name="Prince"/> |
| - | The turns at the C and N termini of b are exposed to an aqueous environment. These turns are | + | The turns at the C and N termini of b are exposed to an aqueous environment. These turns are characterized by a side-chain to main-chain hydrogen bond involving hydrophilic residues.<ref name="Prince"/> |
| - | Turns in the a apoprotein have consequences for oligomerization and pigment binding. The N-terminal portion of a is involved in the binding of a Bchl a pigment, and is submerged in the membrane. | + | Turns in the a apoprotein have consequences for oligomerization and pigment binding. The N-terminal portion of a is involved in the binding of a Bchl a pigment, and is submerged in the membrane. <ref name="Prince"/> |
| - | + | [[Image:Helices_a_b.png|300px|left|thumb|helices | |
| - | [[Image:Helices_a_b.png| | + | are represented by ribbons and the complete pigments are included in a stick representation. image and description from <ref name="Prince"/>).]] |
| - | are represented by ribbons and the complete pigments are included in a stick representation | + | |
'''Membrane interaction''' | '''Membrane interaction''' | ||
| - | The LH2 complex forms a <scene name='91/911263/Lh2_ring/1'>ring within the membrane</scene> and therefore encloses a volume of phospholipid. the bounding a apoprotein trans-membrane helices present an hydrophobic enclosure. click here to se in blue the <scene name='91/911263/Water/1'>soluble water</scene> around the complex. | + | The LH2 complex forms a <scene name='91/911263/Lh2_ring/1'>ring within the membrane</scene> and therefore encloses a volume of phospholipid. the bounding a apoprotein trans-membrane helices present an hydrophobic enclosure. click here to se in blue the <scene name='91/911263/Water/1'>soluble water</scene> around the complex.<ref name="Prince"/> |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | The external surface of LH2 is provided mainly by the inclined <scene name='91/911263/B_apoprotein_ring/1'>b apoproteins</scene>, this surface is very hydrophobic. Portions of B800 and B850 plus a putative second <scene name='91/911263/Carotenoids/1'>independent carotenoid</scene> are exposed within the membrane-spanning region.<ref name="Prince"/> | |
| - | + | ||
| + | unlike other membrane proteins that usually present band of hydrophobic aromatic residues protruding from the external surface of the structure at | ||
| + | the level of membrane emergence, which act as membrane 'anchors', LHII don't present that on its periphery probably due to its necessity of being in physical contact with either LH1 or other LH2 complexes.<ref name="Prince"/> | ||
| + | '''Protein-protein interactions''' | ||
| - | + | The formation of the LH2 complex and therefore the relative dispositions of the pigments is strongly influenced by interactions amongst the pigments | |
| - | + | themselves. They predominantly involve side-chains and are relatively few in number. beta Trans-membrane helices are isolated from each other, and so interact only with pigments.<ref name="Prince"/> | |
| - | + | Within the trans-membrane region, protein-protein contacts are limited to hydrophobic interactions between adjacent a-apoprotein helices. | |
| - | + | the saturated phytyl chain of the aB850 molecule inserts between the adjacent helices, as does the saturated portion of the carotenoid, These minimize and subdivide the direct protein-protein interactions. <ref name="Prince"/> | |
| - | + | ||
| - | + | ||
| - | + | ||
| + | [[Image:A-a_trans-membrane_helix_interaction..png|300px|left|thumb|a-a trans-membrane helix interaction. Pig- | ||
| + | ments and interacting residue side-chains are shown as van der Waals spheres, atoms are coloured green for phytyl chain, yellow for carotenoid, ``key'' residues are cyan, remaining residues are coloured according to atom type; white C, red O. image and description from (<ref name="Prince"/>).]] | ||
| + | in the case of the LHC II the carotenoid present is rhodopin glucoside. | ||
| - | + | [[Image:LHC II .png|400px|right|thumb|LH2 complex: (a) and (b) viewed into the membrane surface; | |
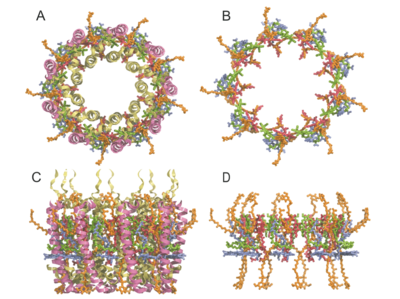
| - | + | (c) and (d) view along the membrane surface. The a-chains are drawn as light-green and b-chain as purple ribbons. | |
| - | < | + | Bacteriochlorophylls are a-B850 (red), b-B850 (green), B800 (blue) and rhodopin glucoside (orange). The cytoplasmic |
| - | + | surface (N terminus) is at the bottom, image and description from <ref name="PAPIZ, Miroslav Z. et al"/>]] | |
| - | + | ||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
| - | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
Contents |
Light Harvesting Complex II
|
introduction
Photosynthesis is the main source of energy used by life on earth nowadays. "The beginning of photosynthesis starts with the absorption of sunlight by an arrangement of photosynthetic pigments embedded into a proteic matrix called the light harvesting (LH) antenna complexes. The excitation energy of photosynthetic pigments is then transferred to the photosynthetic reaction center where it is converted into chemical energy".[1] Photosynthesis is divided in a few steps in a way that the antenna system does not do any chemistry, since it only works by transfering energy in the state of excited electrons between molecules. According to [2] this is only possible due to a weak energetic coupling of the antenna pigments, which are bound to proteins in highly specific associations.
Is it important to notice that this page describes the LHC II of purple bacterias, more specifically the one found in Rhodopseudomonas acidophila, which caries a similiar name but has nothing to do to the LHC II of plants and algae.
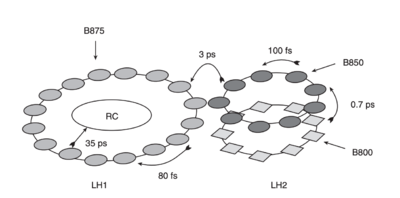
IN general Purple bacteria contain two types of antenna complexes, integral membrane proteins, the light harvesting complex I and II. LHI associates with the reaction center and is always present, constituting part of the "core complex" as explained by [3] . Light harvest complex two, also called LHII is located on the periphery of this core complex and its not always present, since its produced by the bacteria as an acessory complex depending on the availability of light levels encountered by the organism, in order to increase its range of absorption(Zuber & Brunisholz, 1991). Its important to notice that Both types of complex are built on a similar modular principle.
When purple bacteria are grown under anaerobic conditions they incorporate the photosynthetic apparatus described above into invaginated intracytoplasmic phospholipid membranes.[3]
Function
As mentioned light-harvesting complexes are important for Purple bacterial to maximize the spectrum of light available to them, modifying the absorption properties of their chromophores;
In the LHC The proteins help to determine the disposition of the pigments, with, as it will be explained, end up influencing their absorption spectra, together with other factors such as inter-pigment geometries and their interactions with protein and membrane environments.[4]
Pigmentwise, purple bacterias in general contain bacteriochlorophyll a or b, never both and in the case of Rhodopseudomonas acidophila, bacteriochlorophyll a, also abreviated to BChl a, associated with carotenoids, non-covalently bound to two low-molecular-weight hydrophobic apoproteins [3]
It is the principal chlorophyll-type pigment in the majority of anoxygenic photosynthetic bacteria and acomodate several structural changes in comparison to the typical clorophin pigments, specially regarded its symmetry, witch impose effects on its spectral properties.
By clicking is possible to visualize that there are two types of bacteriochlorophylll in different conformations regarding the whole complex, which explains why this pigments can absorb in two different frequencies. We can visualize there are B800 pigments which form a ring parallel to the plane of the membrane that the complex is embedded in. According to the same authors The B800 pigments "are weakly coupled to each other and are at a distance of approximately 21 Å from each other". This independence they keep as molecules answer for their spectral absorption range. The rest of the bacteriochlorophylls can be seen arranged perpendicularly to the plane of the membrane and consist of The pigments that absorb at 850. Each subunit complex has two molecules of bacteriochlorophyll B850 arranged as a closely coupled dimer, which constitute a band of 16 or 18 pigments when the subunit complexes assemble into the LH2 complex. "This band of B850 pigments are all strongly exciton-coupled together, so excitations are effectively delocalized over much or all of the entire band, rather than being effectively localized in a single pigment for a short while and then hopping to another pigment, as is the case with the B800 pigments".
To further understand the difference on absorbance between the bacteriochlorophylls in a more independet state and in a more conjugated state is important to understand the concept of delocalization of energy, which is the extra stability a compound has as a result of having delocalized electrons.[5] " Electron delocalization is also called resonance. Therefore, delocalization energy is also called resonance energy." "Charge delocalization is a stabilizing force because it spreads energy over a larger area rather than keeping it confined to a small area. Since electrons are charges, the presence of delocalized electrons brings extra stability to a system compared to a similar system where electrons are localized."
Since conjugation brings up electron delocalization, it follows that the more extensive the conjugated system, like B850 molecules, the more stable it is, at the same time that its potential energy is lower [5]
Acordig to [2], The spectra of photosynthethic pigments in general can be described by using the “four orbital” model. In this model there are four p molecular orbitals involved, simplified as HOMOs (highest occupied molecular orbitals) and LUMOs ( lowest unoccupied molecular orbitals).
The two lowest-energy transitions are called the Q bands, they are in the visible region of the spectrum and are the most important for the photophysics, and the two higher-energy ones are known as the B bands or Soret bands, and are found in the UV region composing of a large series of electronic transitions.[1] [3].
In order to further understand how the pigments respond to light excitation, its important to visually the molecules x and y axis. By convention, the y molecular axis is defined as the axis passing through the N atoms of rings A and C as show in the figure;
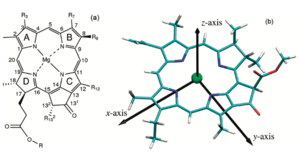
[1]. Acording to [2] "The longest-wavelength transition is invariably polarized along the y axis of the molecule and is therefore known as the Qy transition. This means that the absorption will be strongest if the electric vector of plane-polarized exciting light is parallel to the y molecular axis of the pigment. The exciting light couples to the p electrons of the molecule and rearranges them somewhat during the transition". This Qy transition causes a shift in electron density along the axis and its called a transition dipole. Like other dipole moments, this dipole refers to a difference in charge from one region of a molecule to another. [6]
Energy transfer mechanism
When a Bchl molecule is excited by light, its first excited state lasts for a few nanoseconds, therefore energy transfer within the LH2 complex can occur from the BSOO to the B850 BChl a molecules in just 0.7 ps, transfering the absorbed energy to the reaction centre. Some of the important features that allow this to take place are revealed by its structure, such as the ultrafast depolarization that take place once the energy reaches the B850 molecules(on the 200-300 fs timescale).[3]
The ring of B850 Bchl a molecules acts like a 'storage ring', with the excited state rapidly delocalized over a large area, facilitated by a highly hydrophobic environment which reduces the dielectric constant, allowing coupling over large distances. The energy is then available for transfer from any part of the ring to any neighbouring LHI complex.[3] There are many structural similarities between LHI and LHII, such as the connections between their bacteriochlorophyll to their histidine residues, guaranteeing that their bacteriochlorophyll rings are at the same orientation, which facilitate the energy transfer and create no requirement for the LHI complex to have a special orientation to receive energy from the LH2 ring. The facility of energy transfer between LHII and LHI lead to a further kinetic gains. Studies also have shown that the carotenoid in this LH2 complex acts as an efficient accessory light-harvesting pigment (>50%)7. The excited singlet lifetime of carotenoids is usually less than 10 pS8. Therefore, if energy transfer is to compete successfully with these rapid de-excitation processes, the carotenoid must be located very close to the acceptor bacteriochlorophylls, as seen in the structure. [3]
The carotenoid molecules have an extended conformation and lie generally perpendicular to the plane of the membrane, with close approach (3.4–3.7 Å) to both the B800 and the B850 pigments.
Structure
</StructureSection>==Light Harvesting Complex II==
|
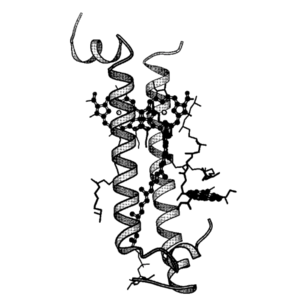
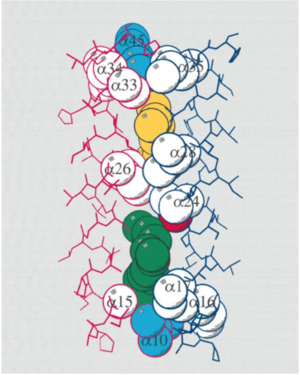
The differences between the LH1 and LH2 complexes reside in their protein/pigment stoichiometry and modes of oligomerization. Structural studies have shown that LH2 complexes are formed from nine , shown in puprle, organized in a ring of inner a (hollow cylinder of radius 18 A) in light blue and outer (outer cylinder of radius 34 A) in dark blue. The a-apoprotein contains 53 amino acids, forming a hollow cylinder of radius 18 A and the b-apoprotein contains 41 amino acids and are arranged radially with the a-apoproteins to form an outer cylinder of radius 34 A. The a-apoprotein helices are parallel to the ninefold axis to within 2°, and the b-apoprotein helices are inclined by 15° relative to this axis [3]
The Bchl a-binding histidines of the a (His 31) and b (His 30) apoproteins face outwards and inwards, respectively, forming a complete ring of 18 overlapping Bchl a molecules. For these molecules, the planes of the bacteriochlorins are parallel to the membrane normal and their centers are approximately 10A from the presumed periplasmic membrane surface. The nine remaining Bchl a molecules are packed between the b-apoprotein helices a further 16.5 A into the membrane with their bacteriochlorin rings parallel to the membrane surface. [7]
The apoprotein subunits
Each apoprotein subunit forms a single helix though out the membrane bilayer with the N-terminus on the cytoplasmic side. In the the N-terminal is approximately 9 A from the presumed membrane surface, and coordinates to the central .
Proceeding from the N-terminal a-helice, a loop involved in the coordination and environ of a Bchl a molecule gives way to a short section of . The 310 helix penetrates the plane of the membrane surface and its linked to a seven-turn transmembrane a-helix, which then links to a two-turn amphipathic helix on periplasmic surface of the membrane. These small terminal helices might act as anchors to the transmembrane helixes perpendicular to the membrane surfaces.[7]
The fold of the is similar, although shorter. Unexpectedly, the only direct interaction between the helices is at the N- and C-terminal ends. Within membrane-buried portions of the helices, interactions are mediated via the pigment molecules or buried water.[7]
The eighteen B850 Bchl a molecules are supported by on the a- and b-apoproteins with their bacteriochlorin rings perpendicular to the membrane surface and associated phytyl chains descending into the hydrophobic core of the assembly. In contrast, the B800 Bchl a molecules have their bacteriochlorin rings aligned parallel to the membrane surface. [7]
The turns at the C and N termini of b are exposed to an aqueous environment. These turns are characterized by a side-chain to main-chain hydrogen bond involving hydrophilic residues.[7]
Turns in the a apoprotein have consequences for oligomerization and pigment binding. The N-terminal portion of a is involved in the binding of a Bchl a pigment, and is submerged in the membrane. [7]
Membrane interaction
The LH2 complex forms a and therefore encloses a volume of phospholipid. the bounding a apoprotein trans-membrane helices present an hydrophobic enclosure. click here to se in blue the around the complex.[7]
The external surface of LH2 is provided mainly by the inclined , this surface is very hydrophobic. Portions of B800 and B850 plus a putative second are exposed within the membrane-spanning region.[7]
unlike other membrane proteins that usually present band of hydrophobic aromatic residues protruding from the external surface of the structure at the level of membrane emergence, which act as membrane 'anchors', LHII don't present that on its periphery probably due to its necessity of being in physical contact with either LH1 or other LH2 complexes.[7]
Protein-protein interactions
The formation of the LH2 complex and therefore the relative dispositions of the pigments is strongly influenced by interactions amongst the pigments themselves. They predominantly involve side-chains and are relatively few in number. beta Trans-membrane helices are isolated from each other, and so interact only with pigments.[7] Within the trans-membrane region, protein-protein contacts are limited to hydrophobic interactions between adjacent a-apoprotein helices. the saturated phytyl chain of the aB850 molecule inserts between the adjacent helices, as does the saturated portion of the carotenoid, These minimize and subdivide the direct protein-protein interactions. [7]
in the case of the LHC II the carotenoid present is rhodopin glucoside.
References
- ↑ 1.0 1.1 1.2 M. Belén Oviedo and Cristián G. Sánchez The Journal of Physical Chemistry A 2011 115 (44), 12280-12285 doi: //doi.org/10.1021/jp203826q
- ↑ 2.0 2.1 2.2 2.3 Blankenship, R. E. Molecular Mechanisms of Photosynthesis; Blackwell Science Ltd.: Oxford, U.K., 2002.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 MCDERMOTT, G. M. et al. Crystal structure of an integral membrane light-harvesting complex from photosynthetic bacteria. Nature, v. 374, n. 6522, p. 517-521, 1995.https://doi.org/10.1038/374517a0
- ↑ 4.0 4.1 PAPIZ, Miroslav Z. et al. The structure and thermal motion of the B800–850 LH2 complex from Rps. acidophila at 2.0 Å resolution and 100 K: new structural features and functionally relevant motions. Journal of molecular biology, v. 326, n. 5, p. 1523-1538, 2003.
- ↑ 5.0 5.1 https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Chemical_Bonding/Valence_Bond_Theory/Delocalization_of_Electrons
- ↑ https://bmc1.utm.utoronto.ca/~vijay/prototype_V12/physChem/molExcit/p08/index.html
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 PRINCE, S. M. et al. Apoprotein structure in the LH2 complex from Rhodopseudomonas acidophila strain 10050: modular assembly and protein pigment interactions. Journal of molecular biology, v. 268, n. 2, p. 412-423, 1997.