User:Isabela de Aquino Zogbi/Sandbox1
From Proteopedia
< User:Isabela de Aquino Zogbi(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
== Dysferlin == | == Dysferlin == | ||
| - | <StructureSection load='4CAI' size='340' side='right' caption='Assymetric Unit structure of | + | <StructureSection load='4CAI' size='340' side='right' caption='Assymetric Unit structure of DysF domain of human dysferlin (pdb code 4CAI)' scene=''> |
==Introduction== | ==Introduction== | ||
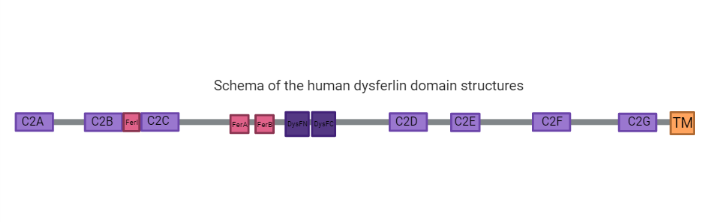
| - | <scene name='91/915204/ | + | <scene name='91/915204/Dysferlin_alphafold/2'>Dysferlin</scene> is a large transmembrane protein of approximately 230kDa encoded by the dysferlin gene (DYSF) highly expressed in striated skeletal and cardiac muscle, but can be found in kidney, placenta, lung and brain tissues. Dysferlin is a protein that belongs to the same family of genes as ''Caenorhabditis elegans'' ferlin, also known as ferlin-like proteins, therefore the name it was given, and can also be known as ferlin 1-like 1 (Fer1L1). It is common to this family the presence of type II transmembrane domains, where the most part of the protein faces de cytoplasm <ref name="omim"> https://www.omim.org/entry/603009?search=dysferlin&highlight=dysferlin </ref>. This protein is critical for repair of muscle membranes after damage and its mutation lead to a progressive muscle dystrophy, since in its absence the membrane tear is not adequately repaired leading to myofiber necrosis and gradual and progressive loss of muscle tissue <ref name="ref1"> de Morrée A., Hensbergen P.J., van Haagen H. H. H. B. M., Dragan I., Deelder A. M., ’t Hoen P. A. C., et al. Proteomic Analysis of the Dysferlin Protein Complex Unveils Its Importance for Sarcolemmal Maintenance and Integrity. PLoS ONE 5(11): e13854 (2010) https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0013854 </ref> <ref name="ref5"/>. The protein rapidly responds to injury with a calcium (Ca2+) influx mechanism which aids the repair. Dysferlin-deficient muscle fibers demonstrate a primary defect in Ca2+-dependent vesicle-mediated membrane repair <ref name="ref5"> Harsini, F. M., A. Bui, A. A., Rice, A. M., Chebrolu, S., Fuson, K. L., Turtoi, A., Bradberry, M., Chapman, E. R., Sutton, R. B. Structural Basis for the Distinct Membrane Binding Activity of the Homologous C2A Domains of Myoferlin and Dysferlin. Journal of Molecular Biology, |
Volume 431, Issue 11, Pages 2112-2126, ISSN 0022-2836 (2019) https://www.sciencedirect.com/science/article/pii/S0022283619301883 </ref>. | Volume 431, Issue 11, Pages 2112-2126, ISSN 0022-2836 (2019) https://www.sciencedirect.com/science/article/pii/S0022283619301883 </ref>. | ||
Current revision
Dysferlin
| |||||||||||
References
- ↑ 1.0 1.1 https://www.omim.org/entry/603009?search=dysferlin&highlight=dysferlin
- ↑ 2.0 2.1 de Morrée A., Hensbergen P.J., van Haagen H. H. H. B. M., Dragan I., Deelder A. M., ’t Hoen P. A. C., et al. Proteomic Analysis of the Dysferlin Protein Complex Unveils Its Importance for Sarcolemmal Maintenance and Integrity. PLoS ONE 5(11): e13854 (2010) https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0013854
- ↑ 3.0 3.1 3.2 3.3 3.4 Harsini, F. M., A. Bui, A. A., Rice, A. M., Chebrolu, S., Fuson, K. L., Turtoi, A., Bradberry, M., Chapman, E. R., Sutton, R. B. Structural Basis for the Distinct Membrane Binding Activity of the Homologous C2A Domains of Myoferlin and Dysferlin. Journal of Molecular Biology, Volume 431, Issue 11, Pages 2112-2126, ISSN 0022-2836 (2019) https://www.sciencedirect.com/science/article/pii/S0022283619301883
- ↑ 4.0 4.1 4.2 4.3 4.4 Sula, A., Cole, A.R., Yeats, C. et al. Crystal structures of the human Dysferlin inner DysF domain. BMC Struct Biol 14, 3 (2014) https://link.springer.com/article/10.1186/1472-6807-14-3
- ↑ Ono, H., Suzuki, N., Kanno, S., Kawahara, G., Izumi, R., Takahashi, T., Kitajima, Y., Osana, S., Nakamura, N., Akiyama, T., Ikeda, K., Shijo, T., Mitsuzawa, S., Nagatomi, R., Araki, N., Yasui, A., Warita, H., Hayashi, Y. K., Miyake, K., Aoki, M. AMPK Complex Activation Promotes Sarcolemmal Repair in Dysferlinopathy. Molecular Therapy, Volume 28, Issue 4, Pages 1133-1153, ISSN 1525-0016,(2020) https://www.sciencedirect.com/science/article/pii/S1525001620300927
- ↑ Abdullah, N., Padmanarayana, M., Marty, N. J., Johnson, C. P. Quantitation of the Calcium and Membrane Binding Properties of the C2 Domains of Dysferlin Biophysical Journal, Volume 106, Issue 2, Pages 382-389, ISSN 0006-3495,(2014) https://www.sciencedirect.com/science/article/pii/S0006349513057536
- ↑ 7.0 7.1 Lek, A., Evesson, F.J., Sutton, R.B., North, K.N. and Cooper, S.T. Ferlins: Regulators of Vesicle Fusion for Auditory Neurotransmission, Receptor Trafficking and Membrane Repair. Traffic, 13: 185-194 (2012) https://onlinelibrary.wiley.com/doi/full/10.1111/j.1600-0854.2011.01267.x
- ↑ Corbalan-Garcia, S., Gómez-Fernández, J. C. Signaling through C2 domains: More than one lipid target. Biochimica et Biophysica Acta (BBA) - Biomembranes, Volume 1838, Issue 6, Pages 1536-1547, ISSN 0005-2736 (2014) https://www.sciencedirect.com/science/article/pii/S0005273614000108?via%3Dihub
- ↑ https://pubs.acs.org/doi/full/10.1021/bi400432f
- ↑ 10.0 10.1 Bansal, D., Campbell, K. P. Dysferlin and the plasma membrane repair in muscular dystrophy. Trends in Cell Biology, Volume 14, Issue 4, Pages 206-213, ISSN 0962-8924 (2004) https://www.sciencedirect.com/science/article/pii/S0962892404000546
- ↑ 11.0 11.1 11.2 Han, R., Campbell, K. P .Dysferlin and muscle membrane repair. Current Opinion in Cell Biology, Volume 19, Issue 4, Pages 409-416, ISSN 0955-0674 (2007) https://www.sciencedirect.com/science/article/pii/S0955067407000993