User:Luize Nóbrega e Silva/Sandbox 1
From Proteopedia
< User:Luize Nóbrega e Silva(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
== Introduction: Thioredoxin system == | == Introduction: Thioredoxin system == | ||
<StructureSection load='1CL0' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='1CL0' size='340' side='right' caption='Caption for this structure' scene=''> | ||
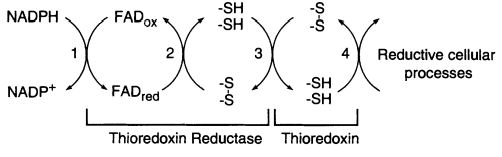
| - | The <scene name='91/914431/1cl0_color/2'>thioredoxin reductase</scene> of E. coli is a member of the flavoenzymes, responsible for catalyzing the reduction of the redox protein thioredoxin, by using NADPH. <ref>PMID:7557016</ref> The <scene name='91/914431/1f6m/3'>Trx System</scene> has its role as a protein disulfide reductase comprising thioredoxin reductase (TrxR), thioredoxin (Trx) and NADPH. Thioredoxin is the main disulfide reductase responsible for keeping intracellular proteins reduced. The redox reactions are catalyzed by thioredoxin reductase (TrxR) by taking electrons from NADPH and transferring them to the | + | The <scene name='91/914431/1cl0_color/2'>thioredoxin reductase</scene> of E. coli is a member of the flavoenzymes, responsible for catalyzing the reduction of the redox protein thioredoxin, by using NADPH. <ref>PMID:7557016</ref> The <scene name='91/914431/1f6m/3'>Trx System</scene> has its role as a protein disulfide reductase comprising thioredoxin reductase (TrxR), thioredoxin (Trx) and NADPH. Thioredoxin is the main disulfide reductase responsible for keeping intracellular proteins reduced. The redox reactions are catalyzed by thioredoxin reductase (TrxR) by taking electrons from NADPH and transferring them to the disulfide of the substrate, Trx. Reducing equivalents are transferred from NADPH to the flavin, generating FAD reduced; then to the TrxR active disulfide, so it can reduce the active disulfide site of the substrate, thioredoxin oxidized. <ref>PMID:20494123</ref> <ref>PMID:11012661</ref> |
The activity of thioredoxin reductase on thioredoxin is importante in E. coli for more than a reason. Despite this enzyme has a conserved role as a high-capacity hydrogen donor systems for reductive enzymes, it also has evolved to specialized functions, such as participating in filamentous E. coli phage assembly and its T7 DNA replication. Furthermore, thioredoxin has demonstrated an important role in defense against oxidative stress or in control of apoptosis. Most of these functions are dependent of the disulfide reductase activity, which is only possible with the NADPH and thioredoxin reductase supply. <ref>PMID:11012661</ref> | The activity of thioredoxin reductase on thioredoxin is importante in E. coli for more than a reason. Despite this enzyme has a conserved role as a high-capacity hydrogen donor systems for reductive enzymes, it also has evolved to specialized functions, such as participating in filamentous E. coli phage assembly and its T7 DNA replication. Furthermore, thioredoxin has demonstrated an important role in defense against oxidative stress or in control of apoptosis. Most of these functions are dependent of the disulfide reductase activity, which is only possible with the NADPH and thioredoxin reductase supply. <ref>PMID:11012661</ref> | ||
[[Image:Trxr reaction.JPG]] | [[Image:Trxr reaction.JPG]] | ||
== Structure == | == Structure == | ||
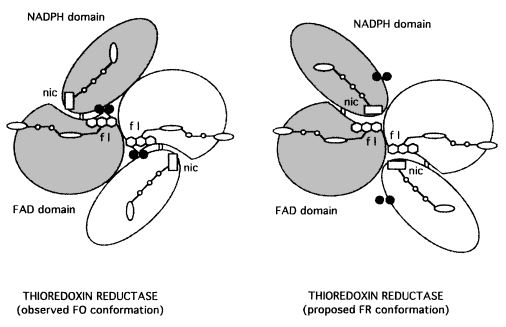
| - | The enzyme thioredoxin reductase from Escherichia coli is a homodimer, in which each monomer contains one <scene name='91/914431/Fad_domain/3'>FAD</scene> and one redox active disulfide. The first part of the FAD binding domain (FAD-i), the NADPH binding domain, and the last part of the FAD binding domain (FAD-2) is formed by the polypeptide chain of each monomer. The connection between FAD and NADPH domains is a two-stranded, antiparallel B-sheet, and each one has a secondary and tertiary structure. <ref>PMID:7557016</ref> | + | The enzyme thioredoxin reductase from Escherichia coli is a homodimer, in which each monomer contains one <scene name='91/914431/Fad_domain/3'>FAD</scene> and one <scene name='91/914431/Active_site/3'>redox active disulfide</scene>. The first part of the FAD binding domain (FAD-i), the NADPH binding domain, and the last part of the FAD binding domain (FAD-2) is formed by the polypeptide chain of each monomer. The connection between FAD and NADPH domains is a two-stranded, antiparallel B-sheet, and each one has a secondary and tertiary structure. <ref>PMID:7557016</ref> |
| - | The redox | + | The redox active disulfide is formed by Cys135 and Cys138, located in the NADPH domain, adjacent to the re side of the flavin ring and interposed between the flavin |
and NADPH binding site. <ref>PMID:7557016</ref> This structure conformation blocks the path of electrons from reduced pyridine nucleotide to the substrate disulfide via the isoalloxazine ring and the redox active disulfide, as it happens in the other member of this enzyme family. That distinction of the NADPH binding site from the flavin happened because of the juxtaposition of the FAD binding and NADPH binding domains, and it can be explained by conformational changes thioredoxin reductase suffer to enable the path of electrons in the catalyze. <ref>PMID:10947986</ref> | and NADPH binding site. <ref>PMID:7557016</ref> This structure conformation blocks the path of electrons from reduced pyridine nucleotide to the substrate disulfide via the isoalloxazine ring and the redox active disulfide, as it happens in the other member of this enzyme family. That distinction of the NADPH binding site from the flavin happened because of the juxtaposition of the FAD binding and NADPH binding domains, and it can be explained by conformational changes thioredoxin reductase suffer to enable the path of electrons in the catalyze. <ref>PMID:10947986</ref> | ||
Current revision
Introduction: Thioredoxin system
| |||||||||||