User:Cézar Santos/Sandbox 1
From Proteopedia
| (One intermediate revision not shown.) | |||
| Line 1: | Line 1: | ||
| - | == | + | == Lon Protease == |
| + | == Introduction == | ||
| + | <StructureSection load='1stp' size='340' side='right' caption='7SXO' scene=''> | ||
Proteins are RNAm-derived essential structures for every living organism in wideworld. They are responsible for maintaining the cell alive and functional, by their actuation intra and extracellular medium, activating the cellular metabolism and maintaining homeostasis. | Proteins are RNAm-derived essential structures for every living organism in wideworld. They are responsible for maintaining the cell alive and functional, by their actuation intra and extracellular medium, activating the cellular metabolism and maintaining homeostasis. | ||
| Line 8: | Line 10: | ||
== Structure == | == Structure == | ||
| - | + | ||
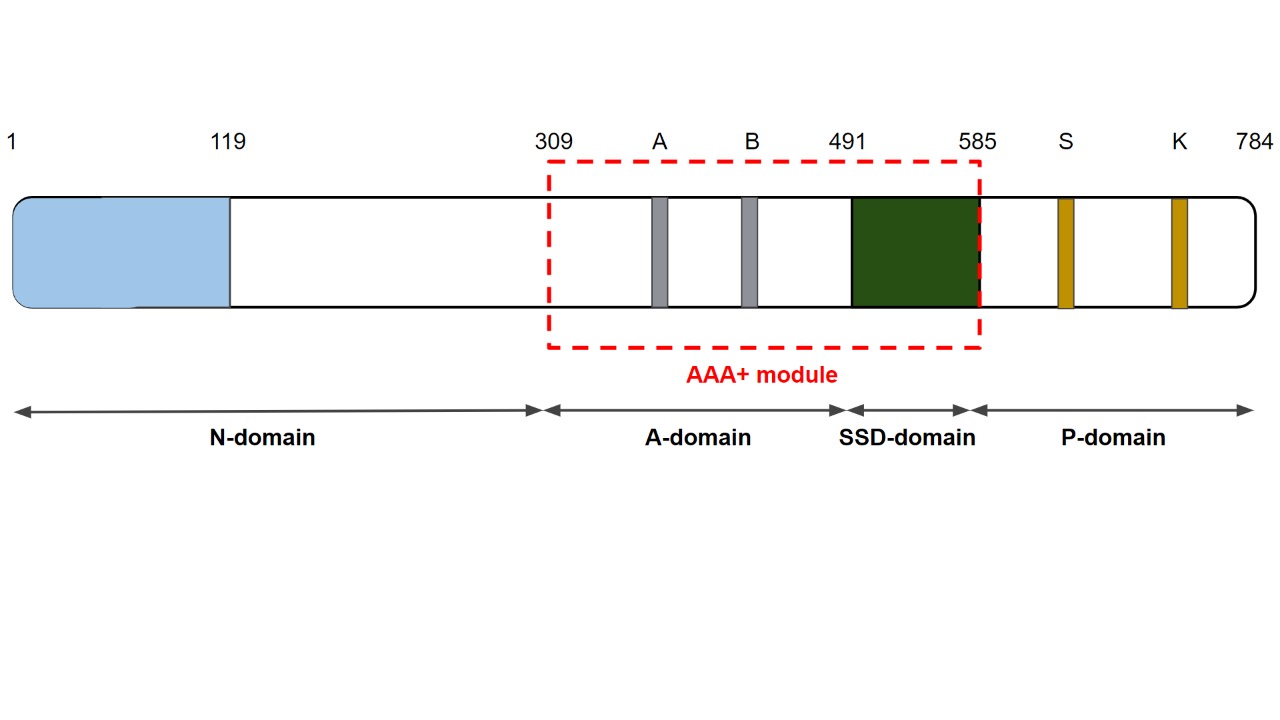
Lon is a protein that contains two catalytic sites, separated by 50aa : a [[protease]], responsible for the unfolding, and [[ATPase]] [[domain]], responsible for degradation of the unfolded proteins[8]. The last contains a AAA+ region, which means that this protein belongs to the group of the ATPases Associated with a variety of cellular Activities. They are normally associated with DNA replication, transcription, membrane fusion, and proteolysis[2]. | Lon is a protein that contains two catalytic sites, separated by 50aa : a [[protease]], responsible for the unfolding, and [[ATPase]] [[domain]], responsible for degradation of the unfolded proteins[8]. The last contains a AAA+ region, which means that this protein belongs to the group of the ATPases Associated with a variety of cellular Activities. They are normally associated with DNA replication, transcription, membrane fusion, and proteolysis[2]. | ||
| Line 15: | Line 17: | ||
The structural organization of the protein is arranged in a series of oligomeric ring-shaped complexes. But it is believed that this state exists in a reversible equilibrium that is dependent on the quantity of ATP present in the substrate[2]. Using electronic microscopy, it was seen that in the presence of ATP, the structure presents as a heptameric-ring with a cavity in the center[3] with flexible subunits[4]. This conformation acts as a machine that mediates folding and degradation of other proteins. However, the absence of nucleotides results in the distortion of the rings, and the presence of leg extrusions. Is believed that this structural asymmetric alteration may be associated with a mechanism by which protein substrates are unfolded and processively translocated to the active site of the protease[2]. | The structural organization of the protein is arranged in a series of oligomeric ring-shaped complexes. But it is believed that this state exists in a reversible equilibrium that is dependent on the quantity of ATP present in the substrate[2]. Using electronic microscopy, it was seen that in the presence of ATP, the structure presents as a heptameric-ring with a cavity in the center[3] with flexible subunits[4]. This conformation acts as a machine that mediates folding and degradation of other proteins. However, the absence of nucleotides results in the distortion of the rings, and the presence of leg extrusions. Is believed that this structural asymmetric alteration may be associated with a mechanism by which protein substrates are unfolded and processively translocated to the active site of the protease[2]. | ||
Although, it is important to emphasize that the atomic model of this protein is still not defined. Initially it was believed that, much like Lon proteins found in other organisms, it presented a hexameric conformation, but using analysis of negative stain electron microscopy of purified PIM1 combined with analytic centrifugation suggested that PIM1 could be a heptamer.[1] | Although, it is important to emphasize that the atomic model of this protein is still not defined. Initially it was believed that, much like Lon proteins found in other organisms, it presented a hexameric conformation, but using analysis of negative stain electron microscopy of purified PIM1 combined with analytic centrifugation suggested that PIM1 could be a heptamer.[1] | ||
| - | + | [[Image:Lon gene.jpeg]] | |
</StructureSection> | </StructureSection> | ||
Current revision
Contents |
Lon Protease
Introduction
| |||||||||||
Function
Most of the functions discovered in this protein occur in S. cerevisiae cells and the main function of Lon protease is protein oxidative degradation and confers stress tolerance. This protease is located in the mitochondrial matrix, due to this organelle being the main source of reactive oxygen species[5]. In the species of yeast Saccharomyces cerevisiae, Lon yet is responsible for stabilizing the genome by regulating gene expression and facilitating the protein complexes in mitochondria[4]. All these works are essentially for maintaining mitochondrial energy metabolism[9].
For proteolysis, there is an interaction between Lon and the chaperone system mtHsp70, so that this system bounding and maintaining the polypeptides with 50-80 amino acids in their structure which will be degraded in an unfolded or soluble state, and then it is use as substrate for Lon during the proteolysis, so this system bounds with Lon P-domain which is located between the lowest and highest ADP-bound subunits called N-domain, as P-domain is responsable for degradating disfunctional protein (fig.1)[9]. It has been shown that Lon is an DNA-binding and interacts directly with gene expression and replication, but more studies are required to understand how this process works. In case of oxidative stress, Lon is critically important to remove soluble polypeptides which were damaged by reactive oxygen species, besides it is responsible for increasing the cellular resistance under external stress conditions[9].
Disregulation
As said, the PIM1 is a highly conserved ATP-dependent Lon protease found in Saccharomyces cerevisiae, and it was shown that its activity declines with age, but also, the deletion of PIM1 shortens the replicative life span[7]. Furthermore, when this protein is disrupted, there is an accumulation of electron-dense inclusion bodies in the mitochondrial matrix space[6].
In this sense, the absence of PIM1 was related to a premature aging of the cells, which is probably caused by the accumulation of oxidized proteins in the cytosol. It is believed that this accumulation acts as an inhibitor of proteasomes, resulting in a lacking of proteasome activity[7].
References
[1]: Yang J, Song AS, Wiseman RL, Lander GC. Cryo-EM structure of hexameric yeast Lon protease (PIM1) highlights the importance of conserved structural elements. J Biol Chem. 2022 Mar;298(3):101694.
[2]: LEE, I.; SUZUKI, C. K. Functional mechanics of the ATP-dependent Lon protease- lessons from endogenous protein and synthetic peptide substrates. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, v. 1784, n. 5, p. 727–735, maio 2008.
[3]: Park SC, Jia B, Yang JK, Van DL, Shao YG, Han SW, Jeon YJ, Chung CH, Cheong GW. Oligomeric structure of the ATP-dependent protease La (Lon) of Escherichia coli. Mol Cells. 2006;21:129–134
[4]: Stahlberg H, Kutejova E, Suda K, Wolpensinger B, Lustig A, Schatz G, Engel A, Suzuki CK. Mitochondrial Lon of Saccharomyces cerevisiae is a ring-shaped protease with seven flexible subunits. Proc Natl Acad Sci U S A. 1999;96:6787–6790.
[5]:Ngo JK, Davies KJ. Mitochondrial Lon protease is a human stress protein. Free Radic Biol Med. 2009 Apr 15;46(8):1042-8. doi: 10.1016/j.freeradbiomed.2008.12.024. Epub 2009 Jan 15. PMID: 19439239; PMCID: PMC3093304.
[6]: SUZUKI, C. K. et al. Requirement for the Yeast Gene LON in Intramitochondrial Proteolysis and Maintenance of Respiration. Science, v. 264, n. 5156, p. 273–276, 8 abr. 1994. Disponível em: <https://www.science.org/doi/10.1126/science.8146662>.
[7]: ERJAVEC, N. et al. Deletion of the mitochondrial Pim1/Lon protease in yeast results in accelerated aging and impairment of the proteasome. Free Radical Biology and Medicine, v. 56, p. 9–16, mar. 2013.
[8]: VAN DIJL, J. M. et al. The ATPase and protease domains of yeast mitochondrial Lon: Roles in proteolysis and respiration-dependent growth. Proceedings of the National Academy of Sciences, v. 95, n. 18, p. 10584–10589, set. 1998.
[9]: Voos W, Pollecker K. The Mitochondrial Lon Protease: Novel Functions off the Beaten Track? Biomolecules. 2020; 10(2):253. https://doi.org/10.3390/biom10020253