Journal:Acta Cryst D:S2059798322008373
From Proteopedia
(Difference between revisions)

| (14 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <StructureSection load='' size='450' side='right' scene=' | + | <StructureSection load='' size='450' side='right' scene='92/920253/Cv/7' caption=''> |
===Structural basis for the transformation of traditional medicine berberine by bacterial nitroreductase=== | ===Structural basis for the transformation of traditional medicine berberine by bacterial nitroreductase=== | ||
| - | <big>Heng Zhang</big> <ref>doi: 10.1107/S2059798322008373</ref> | + | <big>Hai-Ying Wen, Li-Bin Pan, Shu-Rong Ma, Xin-Yu Yang, Jia-Chun Hu, Hai-Fan Zhao, Zeng-Qiang Gao, Yu-Hui Dong, Jian-Dong Jiang, Yan Wang and Heng Zhang</big> <ref>doi: 10.1107/S2059798322008373</ref> |
<hr/> | <hr/> | ||
<b>Molecular Tour</b><br> | <b>Molecular Tour</b><br> | ||
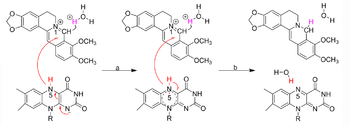
| - | Crystal structure of bacterial nitroreductase (NR) NfsB in complex with the traditional medicine berberine (BBR) showed BBR binds into the active pocket at the NfsB dimer interface. BBR is mainly stabilized by π-stacking interactions with both neighboring aromatic residues and the cofactor FMN. Several well-ordered water molecules neighboring BBR in the active site probably donate protons in conjunction with electron transfer from FMN for BBR reduction. | + | Crystal structure of bacterial nitroreductase (NR) NfsB in complex with the traditional medicine berberine (BBR) showed <scene name='92/920253/Cv/5'>BBR binds into the active pocket at the NfsB dimer interface</scene>. The two subunits of NfsB dimer are shown in green and cyan, respectively. The molecules FMN, BBR and DMSO are shown as state blue, magenta and yellow ball-and-sticks, respectively. BBR is mainly stabilized by <scene name='92/920253/Cv/3'>π-stacking interactions with both neighboring aromatic residues and the cofactor FMN</scene>. Several well-ordered <scene name='92/920253/Cv/4'>water molecules neighboring BBR in the active site</scene> probably donate protons in conjunction with electron transfer from FMN for BBR reduction. Water molecules are shown as red spheres. <scene name='92/920253/Cv/6'>All berberine interactions</scene>. |
| + | [[Image:NRpathway.png|left|350px|thumb|A proposed mechanism of BBR-to-dhBBR conversion by bacterial NRs]] | ||
| + | {{Clear}} | ||
| + | <span class="bg-yellow"><span class="far fa-hand-point-right"></span> Remember to drag the structures with the mouse to rotate them.</span> | ||
<b>References</b><br> | <b>References</b><br> | ||
Current revision
| |||||||||||
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.