We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Potassium Channel
From Proteopedia
(Difference between revisions)
(Undo revision 4316085 by Ann Taylor (Talk)) |
|||
| (3 intermediate revisions not shown.) | |||
| Line 3: | Line 3: | ||
==Function== | ==Function== | ||
| - | [[Potassium Channel]]'''s''' control cell membrane electric potentials by selectively allowing diffusion of K<sup>+</sup> across the membrane.<ref name="Zhou">PMID: 11689936</ref> K<sup>+</sup> Channels extend across the cell membrane, a 40Å thick lipid bilayer which ions cannot cross.<ref name="Doyle">PMID: 9525859</ref> Potassium homeostasis is crucial for nearly all living cells, but is particularly important for the correct function of neurons. Neurons produce electrical impulses known as action potentials, allow for cellular communication processes like neurotransmitter release or to initiate intercellular processes muscle contraction. At the onset of an action potential, sodium ions flood across the plasma membrane of neurons via sodium channels. The change in polarity of the plasma membrane caused by the sodium ion influx inactivates sodium channels. Potassium channels subsequently open allowing the selective diffusion of K<sup>+</sup> ions across the plasma membrane, returning the membrane polarity to neutral. After the action potential has passed, channels recreate the high potassium concentration within the cell in preparation for the next stiumulus.<ref>PMID:12721618 | + | [[Potassium Channel]]'''s''' control cell membrane electric potentials by selectively allowing diffusion of K<sup>+</sup> across the membrane.<ref name="Zhou">PMID: 11689936</ref> K<sup>+</sup> Channels extend across the cell membrane, a 40Å thick lipid bilayer which ions cannot cross.<ref name="Doyle">PMID: 9525859</ref> Potassium homeostasis is crucial for nearly all living cells, but is particularly important for the correct function of neurons. Neurons produce electrical impulses known as action potentials, allow for cellular communication processes like neurotransmitter release or to initiate intercellular processes muscle contraction. At the onset of an action potential, sodium ions flood across the plasma membrane of neurons via sodium channels. The change in polarity of the plasma membrane caused by the sodium ion influx inactivates sodium channels. Potassium channels subsequently open allowing the selective diffusion of K<sup>+</sup> ions across the plasma membrane, returning the membrane polarity to neutral. After the action potential has passed, channels recreate the high potassium concentration within the cell in preparation for the next stiumulus.<ref>PMID:12721618</ref> |
Potassium channels possess two traits that are seemingly mutually exclusive. Firstly, potassium channels have exquisite selectivity, with an amazing 10,000 fold selectivity for K<sup>+</sup> ions over sodium ions. Considering the only difference by which potassium ions can be differentiated from sodium ions is potassium ions’ 1.33Å Pauling radius vs. Sodium’s .95Å radius, the selectivity of potassium channels is remarkable.<ref name="Doyle"/> Second, despite its remarkable selectivity, potassium channels allow for the transfer of K<sup>+</sup> ions across the cell membrane at a rate of nearly 10<sup>8</sup> per second, nearly at the diffusion rate limit.<ref name="Long">PMID: 18004376</ref> Potassium channels are able to achieve these remarkable feats due to its amazing structural architecture, which contains several features which not only can sense the voltage potential across a membrane, but also selectively ferry K<sup>+</sup> ions without any outside energy expenditure. | Potassium channels possess two traits that are seemingly mutually exclusive. Firstly, potassium channels have exquisite selectivity, with an amazing 10,000 fold selectivity for K<sup>+</sup> ions over sodium ions. Considering the only difference by which potassium ions can be differentiated from sodium ions is potassium ions’ 1.33Å Pauling radius vs. Sodium’s .95Å radius, the selectivity of potassium channels is remarkable.<ref name="Doyle"/> Second, despite its remarkable selectivity, potassium channels allow for the transfer of K<sup>+</sup> ions across the cell membrane at a rate of nearly 10<sup>8</sup> per second, nearly at the diffusion rate limit.<ref name="Long">PMID: 18004376</ref> Potassium channels are able to achieve these remarkable feats due to its amazing structural architecture, which contains several features which not only can sense the voltage potential across a membrane, but also selectively ferry K<sup>+</sup> ions without any outside energy expenditure. | ||
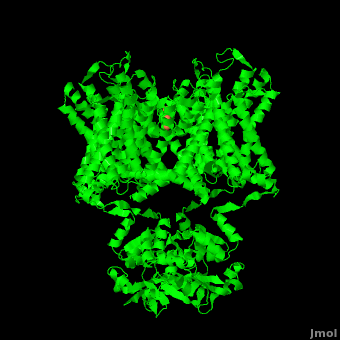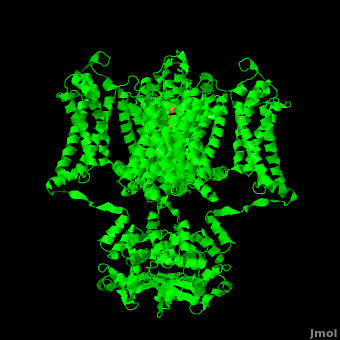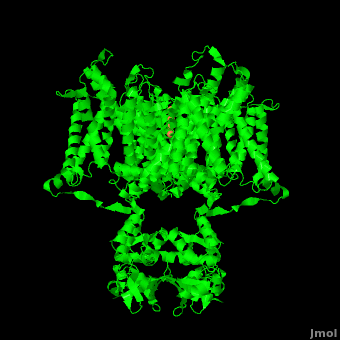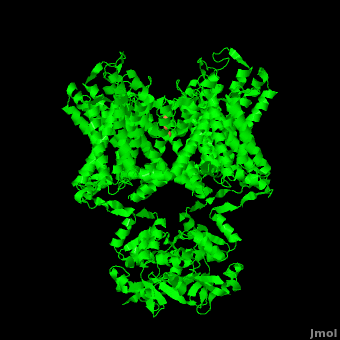
The overall structure of the voltage gated potassium channel can be seen in the image at the left. It is comprised of 4 identical subunits and contains several key features which will be analyzed. Primarily, a <scene name='Potassium_Channel/Trans/3'>transmembrane region</scene> marked between the parallel lines in the figure. This region houses the <scene name='Potassium_Channel/Pore_opening/5'>channel pore</scene>, composed of interwoven helices in a teepee conformation, the all-important <scene name='Potassium_Channel/Selectivity_filter_opening/2'>“selectivity filter”</scene>, providing the channel with its remarkable 10,00 fold selectivity for K<sup>+</sup> ions over Na<sup>+</sup> ions and the <scene name='Potassium_Channel/Voltage_sensors_opening/4'>“voltage sensor”</scene> which is uses well placed arginine and acidic residues to determine the membrane polarity and open/close the channel in response.<ref name="Long"/> | The overall structure of the voltage gated potassium channel can be seen in the image at the left. It is comprised of 4 identical subunits and contains several key features which will be analyzed. Primarily, a <scene name='Potassium_Channel/Trans/3'>transmembrane region</scene> marked between the parallel lines in the figure. This region houses the <scene name='Potassium_Channel/Pore_opening/5'>channel pore</scene>, composed of interwoven helices in a teepee conformation, the all-important <scene name='Potassium_Channel/Selectivity_filter_opening/2'>“selectivity filter”</scene>, providing the channel with its remarkable 10,00 fold selectivity for K<sup>+</sup> ions over Na<sup>+</sup> ions and the <scene name='Potassium_Channel/Voltage_sensors_opening/4'>“voltage sensor”</scene> which is uses well placed arginine and acidic residues to determine the membrane polarity and open/close the channel in response.<ref name="Long"/> | ||
| + | |||
| + | ==Disease== | ||
| + | |||
| + | Mutations in voltage-gated potassium channel KCNC3 have been linked with [[Neurodevelopmental Disorders|neurodevelopmental disorders]] and neurodegeneration.<ref>PMID: 16501573</ref>. [[Amiodarone]] is a potassium channel blocker used in treatment of cardiac dysrhythmias. | ||
==Selectivity Filter and Pore== | ==Selectivity Filter and Pore== | ||
| Line 26: | Line 30: | ||
| - | '''Potassium channels''' (KCh) are subdivided into voltage-gated KCh and calcium-dependent KCh. The latter are subdivided into high- (BK, LKCa), intermediate- and small-conductance KCh (human SK1, rat SK2, SKCa). The T1 domain is a highly conserved N-terminal domain which is responsible for driving the tetramerization of the KCh α subunit. The inward rectifier KCh (IRK) passes current more easily in the inward direction. KCh is activated by | + | '''Potassium channels''' (KCh) are subdivided into voltage-gated KCh and calcium-dependent KCh. The latter are subdivided into high- (BK, LKCa), intermediate- and small-conductance KCh (human SK1, rat SK2, SKCa). The T1 domain is a highly conserved N-terminal domain which is responsible for driving the tetramerization of the KCh α subunit. The inward rectifier KCh (IRK) passes current more easily in the inward direction. KCh is activated by [[Phosphatidylinositol bisphosphate (PIP2)]]. MthK is a calcium-dependent KCh from ''Methanobacterium thermoautrophicum''. |
== 3D structures of Potassium Channels== | == 3D structures of Potassium Channels== | ||
Current revision
| |||||||||||
Additional Resources
References
- ↑ 1.0 1.1 Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001 Nov 1;414(6859):43-8. PMID:11689936 doi:http://dx.doi.org/10.1038/35102009
- ↑ 2.0 2.1 2.2 2.3 Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998 Apr 3;280(5360):69-77. PMID:9525859
- ↑ Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003 May 1;423(6935):33-41. PMID:12721618 doi:http://dx.doi.org/10.1038/nature01580
- ↑ 4.0 4.1 4.2 4.3 Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007 Nov 15;450(7168):376-82. PMID:18004376 doi:http://dx.doi.org/10.1038/nature06265
- ↑ Waters MF, Minassian NA, Stevanin G, Figueroa KP, Bannister JP, Nolte D, Mock AF, Evidente VG, Fee DB, Muller U, Durr A, Brice A, Papazian DM, Pulst SM. Mutations in voltage-gated potassium channel KCNC3 cause degenerative and developmental central nervous system phenotypes. Nat Genet. 2006 Apr;38(4):447-51. Epub 2006 Feb 26. PMID:16501573 doi:ng1758
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, David Canner, Joel L. Sussman, Alexander Berchansky, Ann Taylor