We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Factor Xa
From Proteopedia
(Difference between revisions)
| (6 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
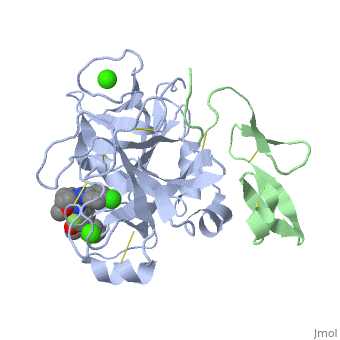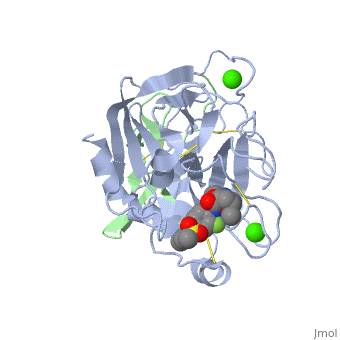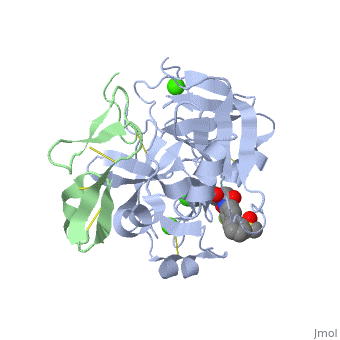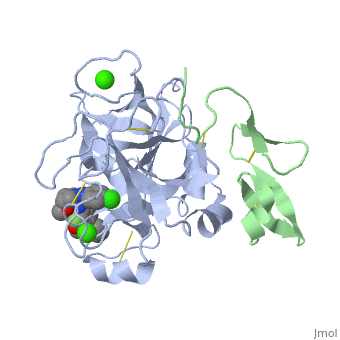
<StructureSection load='2PR3' size='350' side='right' scene='' caption='Human factor X heavy chain (grey) and light chain (green) complex with pyrrolydine derivative inhibitor and Ca+2 ions (green) (PDB code [[2pr3]])'> | <StructureSection load='2PR3' size='350' side='right' scene='' caption='Human factor X heavy chain (grey) and light chain (green) complex with pyrrolydine derivative inhibitor and Ca+2 ions (green) (PDB code [[2pr3]])'> | ||
==Introduction== | ==Introduction== | ||
| - | {{Clear}} | ||
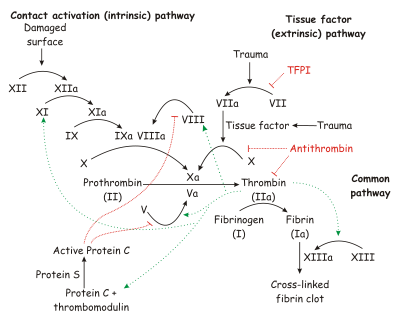
[[Image:Coagulation full.svg.png|left|thumb|450px|The coagulation cascade.]] | [[Image:Coagulation full.svg.png|left|thumb|450px|The coagulation cascade.]] | ||
{{Clear}} | {{Clear}} | ||
| + | |||
'''Factor X''' is a vitamin K-dependent [http://en.wikipedia.org/wiki/Glycoprotein glycoprotein] that is synthesized in the liver. [http://en.wikipedia.org/wiki/Zymogen Zymogen] factor X circulates in plasma as a 2 chain molecule composed of a disulfide linked light chain (Mr = 16500) and heavy chain (Mr = 42,000). Factor X is activated to '''factor Xa''' by cleavage of the activation peptide. This reaction is catalyzed by [http://en.wikipedia.org/wiki/Factor_VIIa factor VIIa]-[http://en.wikipedia.org/wiki/Tissue_factor tissue factor] (extrinsic Xase complex) and [http://en.wikipedia.org/wiki/Factor_ixa factor IXa]-[http://en.wikipedia.org/wiki/Factor_viiia factor VIIIa] (intrinsic Xase complex).<ref name="Greer">Greer, John (2008). ''Wintrobe's Clinical Hematology'', p. 545-546. Lippincott Williams & Wilkins. ISBN 0781765072.</ref> | '''Factor X''' is a vitamin K-dependent [http://en.wikipedia.org/wiki/Glycoprotein glycoprotein] that is synthesized in the liver. [http://en.wikipedia.org/wiki/Zymogen Zymogen] factor X circulates in plasma as a 2 chain molecule composed of a disulfide linked light chain (Mr = 16500) and heavy chain (Mr = 42,000). Factor X is activated to '''factor Xa''' by cleavage of the activation peptide. This reaction is catalyzed by [http://en.wikipedia.org/wiki/Factor_VIIa factor VIIa]-[http://en.wikipedia.org/wiki/Tissue_factor tissue factor] (extrinsic Xase complex) and [http://en.wikipedia.org/wiki/Factor_ixa factor IXa]-[http://en.wikipedia.org/wiki/Factor_viiia factor VIIIa] (intrinsic Xase complex).<ref name="Greer">Greer, John (2008). ''Wintrobe's Clinical Hematology'', p. 545-546. Lippincott Williams & Wilkins. ISBN 0781765072.</ref> | ||
'''Factor Xa''', along with [http://en.wikipedia.org/wiki/Factor_va factor Va], calcium, and a phospholipid membrane surface to form the [http://en.wikipedia.org/wiki/Prothrombinase prothrombinase complex], and cleave [http://en.wikipedia.org/wiki/Prothrombin prothrombin] to its active form, [http://en.wikipedia.org/wiki/Prothrombin thrombin].<ref name="Greer" /> | '''Factor Xa''', along with [http://en.wikipedia.org/wiki/Factor_va factor Va], calcium, and a phospholipid membrane surface to form the [http://en.wikipedia.org/wiki/Prothrombinase prothrombinase complex], and cleave [http://en.wikipedia.org/wiki/Prothrombin prothrombin] to its active form, [http://en.wikipedia.org/wiki/Prothrombin thrombin].<ref name="Greer" /> | ||
| - | + | ==Relevance== | |
| - | + | ||
| - | + | Factor Xa is inhibited by [[Apixaban]] and [[Rivaroxaban]] which are anticoagulant medications. See also [[Anticoagulants]]. | |
==Structure== | ==Structure== | ||
| Line 64: | Line 64: | ||
===General Serine Protease Mechanism=== | ===General Serine Protease Mechanism=== | ||
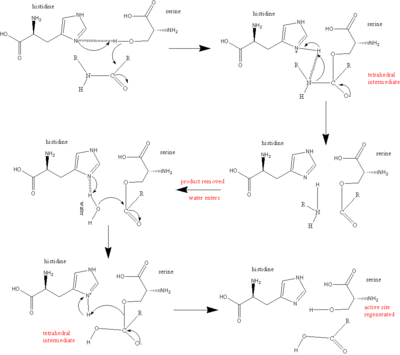
During the acylation half of the reaction His57 acts as a general base to remove a proton from Ser195, allowing it to attack the carbonyl of the peptide bond to be broken within the substrate, to yield the first tetrahedral intermediate. The negative oxygen ion of the tetrahedral intermediate is stabilized through hydrogen bonding with the oxyanion hole (Gly192 and Ser195). Asp102 stabilizes the protonated His57 through hydrogen bonding. His57 protonates the amine of the scissile bond, promoting formation of the acylenzyme and release of the N-terminal portion of the substrate. | During the acylation half of the reaction His57 acts as a general base to remove a proton from Ser195, allowing it to attack the carbonyl of the peptide bond to be broken within the substrate, to yield the first tetrahedral intermediate. The negative oxygen ion of the tetrahedral intermediate is stabilized through hydrogen bonding with the oxyanion hole (Gly192 and Ser195). Asp102 stabilizes the protonated His57 through hydrogen bonding. His57 protonates the amine of the scissile bond, promoting formation of the acylenzyme and release of the N-terminal portion of the substrate. | ||
| - | The deacylation portion repeats the same sequence. A water molecule is deprotonated by His57 and attacks the acyl enzyme, to yielding a second tetrahedral intermediate. Again, the tetrahedral intermediate is stabilized by the oxyanion hole. Upon collapse of the tetrahedral intermediate, the C-terminal portion of the protein is released.<ref name="specificity">PMID:12475199</ref>[[Image:Serine protease mechanism.gif.png| | + | The deacylation portion repeats the same sequence. A water molecule is deprotonated by His57 and attacks the acyl enzyme, to yielding a second tetrahedral intermediate. Again, the tetrahedral intermediate is stabilized by the oxyanion hole. Upon collapse of the tetrahedral intermediate, the C-terminal portion of the protein is released.<ref name="specificity">PMID:12475199</ref> |
| + | [[Image:Serine protease mechanism.gif.png|400px|center|'''Serine protease reaction mechanism''' <ref> www.bmolchem.wisc.edu/</ref> />]] | ||
===Controversial Mechanisms=== | ===Controversial Mechanisms=== | ||
| Line 72: | Line 73: | ||
====Low Barrier Hydrogen Bonds==== | ====Low Barrier Hydrogen Bonds==== | ||
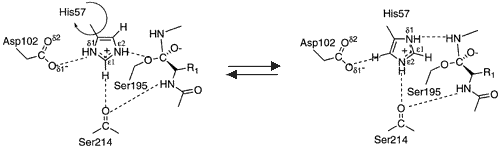
| - | < | + | <scene name='Factor_Xa/Lbhb/1'>Possible LBHB between His57 and Asp102</scene>. The mechanism by which the transition state is stabilized has been the topic of recent debate. Some groups suggest that His57 and Asp102 form and especially strong hydrogen bond, called a [http://en.wikipedia.org/wiki/Low-barrier_hydrogen_bond low barrier hydrogen bond (LBHB)]. They hypothesize that this hydrogen bond could promote formation of the transition state by stabilizing the Asp –His association and enhancing the bascisity of His57. <ref> PMID: 7661899</ref> <ref name="Frey alone">Frey, Perry A. Strong hydrogen bonding in chymotrypsin and other serine proteases. Journal of Physical Organic Chemistry (2004), 17(6-7), 511-520. </ref> This would enhance catalysis in the first step of the reaction. Formation of a LBHB requires a ΔpKa of approximately zero and a donor-to-acceptor distance of less then 2.65 Å for a nitrogen-oxygen pair like His57 and Asp102. Unlike a standard hydrogen bond, in which the hydrogen is located on the donor atom, a hydrogen in a LBHB is located equidistant between the 2 atoms. <ref name="subang"> PMID: 16834383 </ref> In 1998 Kuhn and colleagues published a crystal structure of ''Bacillus lentus'' subtilisn, another serine proetase, with 0.78 Å resolution at pH 5.9. The structure showed a distance of approximately 2.62 Å between the His57 nitrogen and the Asp102 oxygen, suggesting a LBHB. <ref> PMID: 9753430 </ref> |
| - | The mechanism by which the transition state is stabilized has been the topic of recent debate. Some groups suggest that His57 and Asp102 form and especially strong hydrogen bond, called a [http://en.wikipedia.org/wiki/Low-barrier_hydrogen_bond low barrier hydrogen bond (LBHB)]. They hypothesize that this hydrogen bond could promote formation of the transition state by stabilizing the Asp –His association and enhancing the bascisity of His57. <ref> PMID: 7661899</ref> <ref name="Frey alone">Frey, Perry A. Strong hydrogen bonding in chymotrypsin and other serine proteases. Journal of Physical Organic Chemistry (2004), 17(6-7), 511-520. </ref> This would enhance catalysis in the first step of the reaction. Formation of a LBHB requires a ΔpKa of approximately zero and a donor-to-acceptor distance of less then 2.65 Å for a nitrogen-oxygen pair like His57 and Asp102. Unlike a standard hydrogen bond, in which the hydrogen is located on the donor atom, a hydrogen in a LBHB is located equidistant between the 2 atoms. <ref name="subang"> PMID: 16834383 </ref> In 1998 Kuhn and colleagues published a crystal structure of ''Bacillus lentus'' subtilisn, another serine proetase, with 0.78 Å resolution at pH 5.9. The structure showed a distance of approximately 2.62 Å between the His57 nitrogen and the Asp102 oxygen, suggesting a LBHB. <ref> PMID: 9753430 </ref> | + | |
A more recent crystal structure of α-Lytic protease, published in 2006 with 0.82 Å resolution argues against both the his flip mechanism and the presence of a LBHB between His57 and Asp102 (2.755 Å in this structure). Fuhrmann ''et al'' suggests that a LBHB may have been present in the subtilisin strucutre, it is not required for the serine protease mechanism. Instead they state that the chymotrypsin-like proteases may use a network of optimized hydrogen bonds to position the stabilize the tetrahedral intermediate and position the catalytic triad. Ser195 undergoes a shift of ~1Å upon protonation of His57 that destabilizes the His57-Ser195 H-bond. This conformation change would prevent His57 from reprotonating Ser195 leading to regeneration of the substrate.<ref name="subang" /> | A more recent crystal structure of α-Lytic protease, published in 2006 with 0.82 Å resolution argues against both the his flip mechanism and the presence of a LBHB between His57 and Asp102 (2.755 Å in this structure). Fuhrmann ''et al'' suggests that a LBHB may have been present in the subtilisin strucutre, it is not required for the serine protease mechanism. Instead they state that the chymotrypsin-like proteases may use a network of optimized hydrogen bonds to position the stabilize the tetrahedral intermediate and position the catalytic triad. Ser195 undergoes a shift of ~1Å upon protonation of His57 that destabilizes the His57-Ser195 H-bond. This conformation change would prevent His57 from reprotonating Ser195 leading to regeneration of the substrate.<ref name="subang" /> | ||
| Line 87: | Line 87: | ||
**[[1c5m]] - hFX heavy chain catalytic domain + light chain (residues 84-179)<br /> | **[[1c5m]] - hFX heavy chain catalytic domain + light chain (residues 84-179)<br /> | ||
**[[1whe]], [[1whf]] – bFX GLA + EGF-like domains – bovine – NMR<br /> | **[[1whe]], [[1whf]] – bFX GLA + EGF-like domains – bovine – NMR<br /> | ||
| - | **[[ | + | **[[1apo]], [[1ccf]] - bFX EGF-like domain – NMR<br /> |
*Factor Xa complex with inhibitor | *Factor Xa complex with inhibitor | ||
| - | **[[ | + | **[[1v3x]], [[1wu1]], [[2d1j]], [[2ei6]], [[2ei7]], [[2ei8]], [[2p93]], [[2p94]], [[2p95]] – hFX heavy chain residues 16-243 + light chain EGF-like domain + inhibitor<br /> |
| - | + | **[[1fax]], [[1fjs]], [[1g2l]], [[1g2m]], [[1ioe]], [[1iqe]], [[1iqf]], [[1iqg]], [[1iqh]], [[1iqi]], [[1iqj]], [[1iqk]], [[1iql]], [[1iqm]], [[1iqn]], [[1mq5]], [[1mq6]], [[1xka]], [[1xkb]], [[1z6e]], [[2bq6]], [[2bq7]], [[2bqw]], [[2bmg]], [[2bok]], [[2fzz]], [[2g00]], [[2jkh]], [[2p16]], [[2p3t]], [[2p3u]], [[2phb]], [[2pr3]], [[2q1j]], [[2ra0]], [[2vh0]], [[2vh6]], [[2vvc]], [[2vvu]], [[2vvv]], [[2vwl]], [[2vwm]], [[2vwn]], [[2vwo]], [[2w3i]], [[2w3k]], [[2w26]], [[2wyg]], [[2wyj]], [[2xbv]], [[2xbw]], [[2xbx]], [[2xby]], [[2xc0]], [[2xc4]], [[2xc5]], [[2y5f]], [[2y5g]], [[2y5h]], [[2y7x]], [[2y7z]], [[2y80]], [[2y81]], [[2y82]], [[3cs7]], [[3cen]], [[3ens]], [[3ffg]], [[3hpt]], [[3iit]], [[3k9x]], [[3kl6]], [[3kqb]], [[3kqc]], [[3kqd]], [[3kqe]], [[3liw]], [[3m36]], [[3m37]], [[3q3k]], [[3tk5]], [[3tk6]], [[4a7i]], [[4y6d]], [[4y71]], [[4y76]], [[4y79]], [[4y7a]], [[4y7b]] - hFX heavy chain catalytic domain + light chain EGF-like domain + inhibitor<br /> | |
| - | **[[ | + | |
**[[2boh]] - hFX heavy chain catalytic domain (mutant) + light chain EGF-like domain (mutant) + inhibitor<br /> | **[[2boh]] - hFX heavy chain catalytic domain (mutant) + light chain EGF-like domain (mutant) + inhibitor<br /> | ||
| - | **[[ | + | **[[1ezq]], [[1f0r]], [[1f0s]], [[1ksn]], [[1lpg]], [[1lpk]], [[1lpz]], [[1lqd]], [[2cji]], [[2j2u]], [[2j34]], [[2j38]], [[2j4i]], [[2j94]], [[2j95]], [[2uwl]], [[2uwo]], [[2uwp]], [[4zh8]], [[4zha]], [[5k0h]] - hFX heavy chain catalytic domain 235-488 + light chain GLA and EGF-like domains 46-179 + inhibitor<br /> |
**[[1nfu]], [[1nfw]], [[1nfx]], [[1nfy]] - hFX heavy chain catalytic domain + light chain residues 46-240 + inhibitor<br /> | **[[1nfu]], [[1nfw]], [[1nfx]], [[1nfy]] - hFX heavy chain catalytic domain + light chain residues 46-240 + inhibitor<br /> | ||
**[[3sw2]], [[4bti]], [[4btt]], [[4btu]] - hFX heavy chain catalytic domain + light chain residues 84-179 + inhibitor<br /> | **[[3sw2]], [[4bti]], [[4btt]], [[4btu]] - hFX heavy chain catalytic domain + light chain residues 84-179 + inhibitor<br /> | ||
Current revision
| |||||||||||
3D structures of factor Xa
Updated on 08-June-2025
Additional Resources
For additional information, see: Hemophilia
References
- ↑ 1.0 1.1 Greer, John (2008). Wintrobe's Clinical Hematology, p. 545-546. Lippincott Williams & Wilkins. ISBN 0781765072.
- ↑ 2.0 2.1 Department of Chemistry, University of Maine, Orono, ME. http://chemistry.umeche.maine.edu/CHY252/Peptidase3.html
- ↑ Padmanabhan K, Padmanabhan KP, Tulinsky A, Park CH, Bode W, Huber R, Blankenship DT, Cardin AD, Kisiel W. Structure of human des(1-45) factor Xa at 2.2 A resolution. J Mol Biol. 1993 Aug 5;232(3):947-66. PMID:8355279 doi:http://dx.doi.org/10.1006/jmbi.1993.1441
- ↑ Friedman PA, Przysiecki CT. Vitamin K-dependent carboxylation. Int J Biochem. 1987;19(1):1-7. PMID:3106112
- ↑ Vermeer C. Gamma-carboxyglutamate-containing proteins and the vitamin K-dependent carboxylase. Biochem J. 1990 Mar 15;266(3):625-36. PMID:2183788
- ↑ Price PA, Fraser JD, Metz-Virca G. Molecular cloning of matrix Gla protein: implications for substrate recognition by the vitamin K-dependent gamma-carboxylase. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8335-9. PMID:3317405
- ↑ Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995 Apr 7;270(14):7980-7. PMID:7713897
- ↑ Freedman SJ, Blostein MD, Baleja JD, Jacobs M, Furie BC, Furie B. Identification of the phospholipid binding site in the vitamin K-dependent blood coagulation protein factor IX. J Biol Chem. 1996 Jul 5;271(27):16227-36. PMID:8663165
- ↑ Morita T, Jackson CM. Preparation and properties of derivatives of bovine factor X and factor Xa from which the gamma-carboxyglutamic acid containing domain has been removed. J Biol Chem. 1986 Mar 25;261(9):4015-23. PMID:3512564
- ↑ Muskavitch MA, Hoffmann FM. Homologs of vertebrate growth factors in Drosophila melanogaster and other invertebrates. Curr Top Dev Biol. 1990;24:289-328. PMID:2116263
- ↑ Ohlin AK, Linse S, Stenflo J. Calcium binding to the epidermal growth factor homology region of bovine protein C. J Biol Chem. 1988 May 25;263(15):7411-7. PMID:3259233
- ↑ Selander-Sunnerhagen M, Ullner M, Persson E, Teleman O, Stenflo J, Drakenberg T. How an epidermal growth factor (EGF)-like domain binds calcium. High resolution NMR structure of the calcium form of the NH2-terminal EGF-like domain in coagulation factor X. J Biol Chem. 1992 Sep 25;267(27):19642-9. PMID:1527084
- ↑ Persson E, Hogg PJ, Stenflo J. Effects of Ca2+ binding on the protease module of factor Xa and its interaction with factor Va. Evidence for two Gla-independent Ca(2+)-binding sites in factor Xa. J Biol Chem. 1993 Oct 25;268(30):22531-9. PMID:8226763
- ↑ Persson E, Selander M, Linse S, Drakenberg T, Ohlin AK, Stenflo J. Calcium binding to the isolated beta-hydroxyaspartic acid-containing epidermal growth factor-like domain of bovine factor X. J Biol Chem. 1989 Oct 5;264(28):16897-904. PMID:2789221
- ↑ Hopfner KP, Kopetzki E, Kresse GB, Bode W, Huber R, Engh RA. New enzyme lineages by subdomain shuffling. Proc Natl Acad Sci U S A. 1998 Aug 18;95(17):9813-8. PMID:9707558
- ↑ Factor X. Wikipedia
- ↑ Serine Protease. Wikipedia
- ↑ 18.0 18.1 Hedstrom L. Serine protease mechanism and specificity. Chem Rev. 2002 Dec;102(12):4501-24. PMID:12475199
- ↑ 19.0 19.1 Rai R, Sprengeler PA, Elrod KC, Young WB. Perspectives on factor Xa inhibition. Curr Med Chem. 2001 Feb;8(2):101-19. PMID:11172669
- ↑ www.bmolchem.wisc.edu/
- ↑ 21.0 21.1 Bachovchin, W. Contributions of NMR spectroscopy to the study of hydrogen bonds in serine protease active sites. Magnetic Resonance in Chemistry; (2001); 39(Spec. Issue); 199-213.
- ↑ Bachovchin WW. 15N NMR spectroscopy of hydrogen-bonding interactions in the active site of serine proteases: evidence for a moving histidine mechanism. Biochemistry. 1986 Nov 18;25(23):7751-9. PMID:3542033
- ↑ Brady K, Wei AZ, Ringe D, Abeles RH. Structure of chymotrypsin-trifluoromethyl ketone inhibitor complexes: comparison of slowly and rapidly equilibrating inhibitors. Biochemistry. 1990 Aug 21;29(33):7600-7. PMID:2271520
- ↑ Frey PA, Whitt SA, Tobin JB. A low-barrier hydrogen bond in the catalytic triad of serine proteases. Science. 1994 Jun 24;264(5167):1927-30. PMID:7661899
- ↑ Frey, Perry A. Strong hydrogen bonding in chymotrypsin and other serine proteases. Journal of Physical Organic Chemistry (2004), 17(6-7), 511-520.
- ↑ 26.0 26.1 Fuhrmann CN, Daugherty MD, Agard DA. Subangstrom crystallography reveals that short ionic hydrogen bonds, and not a His-Asp low-barrier hydrogen bond, stabilize the transition state in serine protease catalysis. J Am Chem Soc. 2006 Jul 19;128(28):9086-102. PMID:16834383 doi:http://dx.doi.org/10.1021/ja057721o
- ↑ Kuhn P, Knapp M, Soltis SM, Ganshaw G, Thoene M, Bott R. The 0.78 A structure of a serine protease: Bacillus lentus subtilisin. Biochemistry. 1998 Sep 29;37(39):13446-52. PMID:9753430 doi:10.1021/bi9813983
Proteopedia Page Contributors and Editors (what is this?)
Jacqueline Gertz, Michal Harel, Alexander Berchansky, David Canner, Jaime Prilusky