Sandbox Reserved 1780
From Proteopedia
(Difference between revisions)
| (57 intermediate revisions not shown.) | |||
| Line 3: | Line 3: | ||
<StructureSection load= <StructureSection load='Edited_7utz.pse' size='340' side='right' caption='Thyroid stimulating hormone receptor bound to thyroid stimulating hormone' scene='95/952708/Tshr_general_structure/3'> | <StructureSection load= <StructureSection load='Edited_7utz.pse' size='340' side='right' caption='Thyroid stimulating hormone receptor bound to thyroid stimulating hormone' scene='95/952708/Tshr_general_structure/3'> | ||
| - | == Active and Inactive Form == | + | === Active and Inactive Form === |
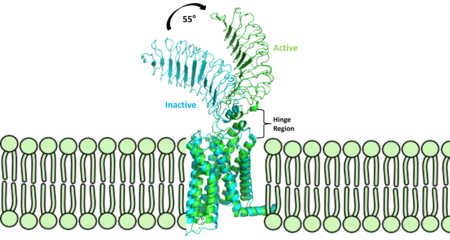
| - | [[Image: | + | [[Image:Finalmorphpic2.png|450 px|right|thumb|Figure 2: Inactive form of the thyrotropin receptor shown in blue (PDB: 7T9M). Active form of the thyrotropin receptor shown in green (PDB: 7T9I).]] |
| - | The TSHR protein exists in two states | + | The TSHR protein exists in dynamic equilibrium between two states: active and inactive (Figure 2). <scene name='95/952708/Tsh_7t9i/2'>TSH</scene> will bind and keep the active state in the up position as a result of clashes between bound TSH and the cell membrane.<ref name="Faust" />. <scene name='95/952708/Tsh_7t9i/4'>Glycolysations of an ASN52 residue</scene> cause this clash on the <scene name='95/952707/Tsh_7t9i/1'>α-subunit of TSH</scene>. |
| - | == TSHR Agonists and | + | == TSHR Agonists and Antagonists == |
| - | Chemical [https://en.wikipedia.org/wiki/Agonist agonists] are found in many living systems and serve as a way to activate receptors or pathways that are necessary for a wide array of biological processes. Chemical [https://en.wikipedia.org/wiki/ | + | Chemical [https://en.wikipedia.org/wiki/Agonist agonists] are found in many living systems and serve as a way to activate receptors or pathways that are necessary for a wide array of biological processes. Chemical [https://en.wikipedia.org/wiki/Receptor_antagonist antagonists] block or inhibit biological processes. Different types of agonists/antagonists exist within the body including hormones, antibodies, and neurotransmitters. The body naturally produces autoantibodies that can act as agonists and mimic the activating mechanism of the natural hormone leading to disease.<ref name="Miguel"> doi:10.1677/JME-08-0152</ref>. |
| - | + | ||
===M22 Agonist=== | ===M22 Agonist=== | ||
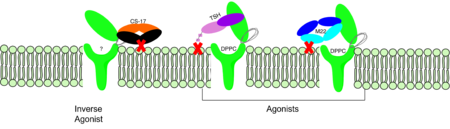
| - | M22 is a [https://en.wikipedia.org/wiki/Monoclonal_antibody monoclonal antibody] that | + | <scene name='95/952708/M22_edited/3'>M22</scene> is a [https://en.wikipedia.org/wiki/Monoclonal_antibody monoclonal antibody] that is produced by patients with [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease Graves' Disease]. In Graves' disease, autoantibodies mimic TSH function and cause thyroid overactivity. <ref name="Miguel"> doi:10.1677/JME-08-0152</ref>. Grave's Disease is an autoimmune disease that is a result of hyperthyroidism, where too much TSH is being produced. This disease [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease effects 1 in 100 Americans and especially women or people older than 30 years of age]. The M22 [https://en.wikipedia.org/wiki/Autoantibody autoantibody] activates TSHR by causing a membrane clash with the ECD and the cell membrane, keeping the TSHR in the active state by preventing the TSHR from rotating to the inactive state (Figure 3). M22 mimics TSH activation of TSHR, and is a potent activator for intracellular signaling. <ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> Although M22 binds in a similar manner to TSH, M22 does not interact with the hinge region when bound to TSHR.<ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> These findings show that the hinge region is not necessary for the activation of TSHR, and leads to the discovery of other methods of activation. [[Image:Agonist pic.png|450 px|right|thumb|Figure 3: Agonist and antagonist drugs for activating or inactivating the TSHR protein. Here the membrane clashes are demonstrated on TSHR with different agonists attached. CS-17 is orange, TSH is purple, and M22 is blue in the figure. The TSHR protein is green and embedded in the protein.]] |
===CS-17 Inverse Agonist=== | ===CS-17 Inverse Agonist=== | ||
| + | <scene name='95/952708/Cs17/1'>CS-17</scene> is a [https://en.wikipedia.org/wiki/Monoclonal_antibody monoclonal antibody] that acts as an inverse agonist for TSHR constitutive activity. <ref name= "Chen et al.">Chen, C.-R., McLachlan, S. M., & Rapoport, B. (2007). Suppression of thyrotropin receptor constitutive activity by a monoclonal antibody with inverse agonist activity. Endocrinology, 148(5), 2375–2382. https://doi.org/10.1210/en.2006-1754</ref>. An example of a disease caused by inverse agonists is [https://www.mayoclinic.org/diseasesconditions/hypothyroidism/symptomscauses/syc20350284#:~:text=Hypothyroidism%20happens%20when%20the%20thyroid,symptoms%20in%20its%20early%20stages hypothyroidism]. The most common cause of hypothyroidism is [https://www.mayoclinic.org/diseasesconditions/hypothyroidism/symptomscauses/syc20350284#:~:text=Hypothyroidism%20happens%20when%20the%20thyroid,symptoms%20in%20its%20early%20stages Hashimoto’s disease]. Without enough TSH to bind TSHR, the pathway remains inactive and thus metabolic processes are inhibited in this pathway. CS-17 interacts with the ECD of the TSHR protein on the | ||
| + | <scene name='95/952708/Cs17/2'>convex side</scene> of the LRRD, suppressing TSHR function by keeping the receptor in the inactive state (Figure 3). Clash of bound CS-17 with the cell membrane locks TSHR in the inactive form. This type of inhibition is uncommon and is a promising mechanism for future drug design and research to combat hypothyroidism.<ref name="Chen et al.">Chen, C.-R., McLachlan, S. M., & Rapoport, B. (2007). Suppression of thyrotropin receptor constitutive activity by a monoclonal antibody with inverse agonist activity. Endocrinology, 148(5), 2375–2382. https://doi.org/10.1210/en.2006-1754</ref>. | ||
| + | |||
| + | |||
| - | [[Image:Agonist pic.png|450 px|right|thumb|Figure 2: Agonist and antagonist drugs for activating or inactivating the TSHR protein.]] | ||
| - | ==Introduction== The Thyroid-stimulating hormone receptor (TSHR) is a G protein-coupled receptor (GPCR) that plays a crucial role in regulating the function of the thyroid gland. TSHR is a transmembrane receptor located on the surface of thyroid follicular cells, which are responsible for producing thyroid hormones. TSHR is activated by Thyroid-stimulating hormone (TSH) and triggers a signaling cascade that results in the production and secretion of thyroid hormones. Dysregulation of TSHR can lead to a variety of thyroid disorders, including hyperthyroidism and hypothyroidism. | ||
| - | ==Structure== The TSHR protein is a large glycoprotein consisting of 764 amino acids. It is composed of three main domains: the extracellular domain, the transmembrane domain, and the intracellular domain. The extracellular domain of TSHR contains a leucine-rich repeat (LRR) domain, which is responsible for binding to TSH. The transmembrane domain is composed of seven alpha-helices, which span the lipid bilayer of the cell membrane. The intracellular domain is responsible for activating downstream signaling cascades upon TSH binding. | ||
| - | [[File:PDB 1hyt.png|thumb|TSHR protein structure from PDB 1HYT showing the extracellular, transmembrane, and intracellular domains. Click on specific regions to see more details. [[Scene Authoring Tools]] can be used to create hyperlinks.]] | ||
| - | ==Agonists== Several TSHR agonists have been identified, including [[M22]] and [[CS-17]]. M22 is a monoclonal antibody that binds to the extracellular domain of TSHR and mimics the action of TSH, leading to increased production and secretion of thyroid hormones. CS-17 is a small molecule that also binds to the extracellular domain of TSHR and activates downstream signaling pathways. | ||
| - | ==Ligands== Recent studies have identified other compounds that can bind to TSHR and modulate its activity. [[ML109]] is a small molecule that can interact with TSHR and modulate its activity, but the precise mechanism of action is still unknown. [[Dipalmitoylphosphatidylcholine|True DPPC]], a phospholipid found in lung surfactant, has also been shown to bind to TSHR and regulate its activity. However, the physiological relevance of this interaction is still unclear. | ||
| - | ==Function== The TSHR protein plays a critical role in regulating the production and secretion of thyroid hormones. When TSH binds to TSHR on the surface of thyroid follicular cells, it activates a signaling cascade that leads to the production and secretion of thyroid hormones. This process is regulated by a negative feedback loop, where increased levels of thyroid hormones in the bloodstream inhibit the production and secretion of TSH from the pituitary gland. | ||
| - | ==Clinical significance== Dysregulation of TSHR can lead to a variety of thyroid disorders. Hyperthyroidism, a condition characterized by an overactive thyroid gland, can be caused by the overstimulation of TSHR by TSH or TSHR agonists. This can result in an excess of thyroid hormones in the bloodstream, leading to symptoms such as weight loss, rapid heartbeat, and tremors. Hypothyroidism, a condition characterized by an underactive thyroid gland, can be caused by the decreased stimulation of TSHR by TSH. This can result in a deficiency of thyroid hormones in the bloodstream, leading to symptoms such as fatigue, weight gain, and cold intolerance. In addition, mutations in the TSHR gene can lead to inherited thyroid disorders. | ||
| - | ==References== {{reflist|30em}} | ||
| - | ==External links== *[https://proteopedia.org/wiki/index.php/TSH_Receptor TSH Receptor] on Proteopedia. | ||
| - | [[Category:Proteins]] [[Category:G protein-coupled receptors]] | ||
Current revision
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Faust B, Billesbolle CB, Suomivuori CM, Singh I, Zhang K, Hoppe N, Pinto AFM, Diedrich JK, Muftuoglu Y, Szkudlinski MW, Saghatelian A, Dror RO, Cheng Y, Manglik A. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05159-1. doi:, 10.1038/s41586-022-05159-1. PMID:35940205 doi:http://dx.doi.org/10.1038/s41586-022-05159-1
- ↑ 2.0 2.1 Nunez Miguel R, Sanders J, Chirgadze DY, Furmaniak J, Rees Smith B. Thyroid stimulating autoantibody M22 mimics TSH binding to the TSH receptor leucine rich domain: a comparative structural study of protein-protein interactions. J Mol Endocrinol. 2009 May;42(5):381-95. Epub 2009 Feb 16. PMID:19221175 doi:10.1677/JME-08-0152
- ↑ 3.0 3.1 Chen, C.-R., McLachlan, S. M., & Rapoport, B. (2007). Suppression of thyrotropin receptor constitutive activity by a monoclonal antibody with inverse agonist activity. Endocrinology, 148(5), 2375–2382. https://doi.org/10.1210/en.2006-1754