Sandbox Reserved 1774
From Proteopedia
(Difference between revisions)
| (3 intermediate revisions not shown.) | |||
| Line 39: | Line 39: | ||
Of the TSHR hinge subregions, the p10 peptide is noteworthy because it serves as an internal agonist of TSHR activation <ref name="Bruser" />. Specifically, an <scene name='95/952702/Ionic_interaction/4'>ionic interaction</scene> between K660 in TM helix 7 and E409 in the p10 region stabilizes TSHR in the active conformation. If this interaction is disrupted with an E409A mutation, diminished receptor activation and TSH potency is observed <ref name="Faust" />. Other mutations in the p10 are poorly tolerated in both TSHR and other glycoprotein hormone receptors <ref name="Faust" />. | Of the TSHR hinge subregions, the p10 peptide is noteworthy because it serves as an internal agonist of TSHR activation <ref name="Bruser" />. Specifically, an <scene name='95/952702/Ionic_interaction/4'>ionic interaction</scene> between K660 in TM helix 7 and E409 in the p10 region stabilizes TSHR in the active conformation. If this interaction is disrupted with an E409A mutation, diminished receptor activation and TSH potency is observed <ref name="Faust" />. Other mutations in the p10 are poorly tolerated in both TSHR and other glycoprotein hormone receptors <ref name="Faust" />. | ||
| - | Beyond the p10 region, the active conformation of TSHR is also stabilized by a <scene name='95/952702/Hydrophobic_interaction/3'>hydrophobic interaction</scene> between Y279 in the hinge helix and I486 in ECL region 1 <ref name="Faust" />. If this interaction is disrupted with an I496F mutation, constitutive receptor activation and decreased sensitivity to the TSH ligand. This receptor overactivation is attributed to over-stabilization of the hydrophobic interaction by the bulkier phenylalanine <ref name="Faust" />. In this way, TSHR function is directly affected by stabilizing interactions in the hinge. | + | Beyond the p10 region, the active conformation of TSHR is also stabilized by a <scene name='95/952702/Hydrophobic_interaction/3'>hydrophobic interaction</scene> between Y279 in the hinge helix and I486 in ECL region 1 <ref name="Faust" />. If this interaction is disrupted with an I496F mutation, the result is constitutive receptor activation and decreased sensitivity to the TSH ligand. This receptor overactivation is attributed to over-stabilization of the hydrophobic interaction by the bulkier phenylalanine <ref name="Faust" />. In this way, TSHR function is directly affected by stabilizing interactions in the hinge. |
Rather than stabilization, two disulfide bonds in the hinge region are responsible for constraining the movement of the hinge <ref name="Kleinau" />. The <scene name='95/952702/P10_hinge_disulfide/5'>first disulfide bridge</scene> connects the hinge helix to the linker region, and the <scene name='95/952702/Linker_hinge_disulfide/4'>second disulfide bridge</scene> connects the hinge helix to the p10 region <ref name="Duan" />. By directly linking parts of the hinge, the disulfide bonds serve to keep the ECD and TMD in close proximity while allowing the domains to move relative to one another <ref name="Kleinau" />. | Rather than stabilization, two disulfide bonds in the hinge region are responsible for constraining the movement of the hinge <ref name="Kleinau" />. The <scene name='95/952702/P10_hinge_disulfide/5'>first disulfide bridge</scene> connects the hinge helix to the linker region, and the <scene name='95/952702/Linker_hinge_disulfide/4'>second disulfide bridge</scene> connects the hinge helix to the p10 region <ref name="Duan" />. By directly linking parts of the hinge, the disulfide bonds serve to keep the ECD and TMD in close proximity while allowing the domains to move relative to one another <ref name="Kleinau" />. | ||
| Line 47: | Line 47: | ||
The thyroid stimulating hormone <scene name='95/952703/Tsh-ecd/4'>binds to the extracellular domain</scene> by complementary shape<ref name="Duan" />. The ECD is curved and compliments the curvature of TSH similar to how a baseball fits into a glove. Several key ionic interactions between the TSH and TSHR also occur in the <scene name='95/952703/Seatbelt/4'>seat belt region of TSH</scene>. The seatbelt region is located in the beta subunit of the TSH. <scene name='95/952703/Tsh-tshr_itxn-3/5'>The first ionic interaction</scene> is Glu118 from TSH and Lys58 from the ECD.<scene name='95/952703/Tsh-tshr_itxn-2/4'>The second interaction</scene> is between Asp111 from the TSH and Lys209 from the ECD. These interactions form salt bridges between the ECD and the TSH which allows for specificity of binding for TSH to TSHR <ref name="Duan" /><ref name="Faust" />. | The thyroid stimulating hormone <scene name='95/952703/Tsh-ecd/4'>binds to the extracellular domain</scene> by complementary shape<ref name="Duan" />. The ECD is curved and compliments the curvature of TSH similar to how a baseball fits into a glove. Several key ionic interactions between the TSH and TSHR also occur in the <scene name='95/952703/Seatbelt/4'>seat belt region of TSH</scene>. The seatbelt region is located in the beta subunit of the TSH. <scene name='95/952703/Tsh-tshr_itxn-3/5'>The first ionic interaction</scene> is Glu118 from TSH and Lys58 from the ECD.<scene name='95/952703/Tsh-tshr_itxn-2/4'>The second interaction</scene> is between Asp111 from the TSH and Lys209 from the ECD. These interactions form salt bridges between the ECD and the TSH which allows for specificity of binding for TSH to TSHR <ref name="Duan" /><ref name="Faust" />. | ||
| - | Other key interactions that determine the specificity of binding are <scene name='95/952703/Tsh-tshr_itxn-4/4'>polar and nonpolar interactions</scene> between TSH and helix 1. Helix 1 contains several polar residues that interact with surrounding nonpolar residues like Leu62 and Phe17. Positively charged Arg54 was also seen to interact with Helix 1. These interactions increase the activation potency and help activate the | + | Other key interactions that determine the specificity of binding are <scene name='95/952703/Tsh-tshr_itxn-4/4'>polar and nonpolar interactions</scene> between TSH and helix 1. Helix 1 contains several polar residues that interact with surrounding nonpolar residues like Leu62 and Phe17. Positively charged Arg54 was also seen to interact with Helix 1. These interactions increase the activation potency and help activate the rotation of the hinge region <ref name="Duan" /><ref name="Faust" />. |
=== Ligand Regulation of Signaling Activation === | === Ligand Regulation of Signaling Activation === | ||
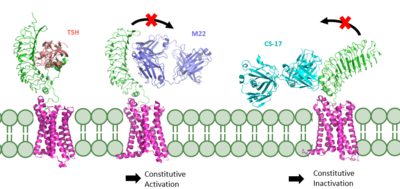
[[Image:conformation1.png|400px|right|thumb|'''Figure 2''' TSHR in active and inactive binding states. '''Left''' is TSH bound to TSHR ([https://www.rcsb.org/structure/7T9I 7T9I]). '''Middle''' is M22 bound to TSHR ([https://www.rcsb.org/structure/7T9N 7T9N]). '''Right''' is CS-17 bound to TSHR ([https://www.rcsb.org/structure/7T9M 7T9M]).]] | [[Image:conformation1.png|400px|right|thumb|'''Figure 2''' TSHR in active and inactive binding states. '''Left''' is TSH bound to TSHR ([https://www.rcsb.org/structure/7T9I 7T9I]). '''Middle''' is M22 bound to TSHR ([https://www.rcsb.org/structure/7T9N 7T9N]). '''Right''' is CS-17 bound to TSHR ([https://www.rcsb.org/structure/7T9M 7T9M]).]] | ||
| - | The interactions between the TSH ligand and the TSHR receptor have significant consequences for disease states as antibodies can stabilize TSHR in the fully active or inactive state<ref name="Faust" />. In the image shown to the right are three different states of TSHR. In contrast to the native TSH conformation, the autoimmune antibody, [https://www.creativebiolabs.net/Anti-TSHR-Antibody-24960.htm M22], bound to TSHR (middle) locks the receptor in the upright state and prevents transition to the down state because of steric clash with the membrane. This conformation causes constitutive activation and elevated levels of thyroid hormones found in a person with Grave's disease<ref name="Faust" />. In contrast to TSH and M22 binding, antibody [https://pubmed.ncbi.nlm.nih.gov/19299457/ CS-17] binds to the TSHR and locks it in the down, inactive conformation. This prevents the signaling cascade to translation and causes constitutive inactivation <ref name="Faust" />. | + | The interactions between the TSH ligand and the TSHR receptor have significant consequences for disease states as antibodies can stabilize TSHR in the fully active or inactive state<ref name="Faust" />. In the image shown to the right are three different states of TSHR. In contrast to the native TSH conformation on the left, the autoimmune antibody, [https://www.creativebiolabs.net/Anti-TSHR-Antibody-24960.htm M22], bound to TSHR (middle) locks the receptor in the upright state and prevents transition to the down state because of steric clash with the membrane. This conformation causes constitutive activation and elevated levels of thyroid hormones found in a person with Grave's disease<ref name="Faust" />. In contrast to TSH and M22 binding, antibody [https://pubmed.ncbi.nlm.nih.gov/19299457/ CS-17] (right) binds to the TSHR and locks it in the down, inactive conformation. This prevents the signaling cascade to translation and causes constitutive inactivation <ref name="Faust" />. |
These different ways to active and inactive TSHR could represent potential therapies for someone with Grave's disease or other thyroid-related diseases with overactive TSH binding. Whereas current therapies target T3/T4 synthesis or destroy the gland using artificial hormones, these diseases could instead be targeted with something like CS-17 which would compete with M22 and TSH to lessen overactivation<ref name="Faust" />. | These different ways to active and inactive TSHR could represent potential therapies for someone with Grave's disease or other thyroid-related diseases with overactive TSH binding. Whereas current therapies target T3/T4 synthesis or destroy the gland using artificial hormones, these diseases could instead be targeted with something like CS-17 which would compete with M22 and TSH to lessen overactivation<ref name="Faust" />. | ||
Current revision
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
| |||||||||||