Sandbox Reserved 1769
From Proteopedia
(Difference between revisions)
| (5 intermediate revisions not shown.) | |||
| Line 26: | Line 26: | ||
=== Mechanism of Bile Salt Uptake === | === Mechanism of Bile Salt Uptake === | ||
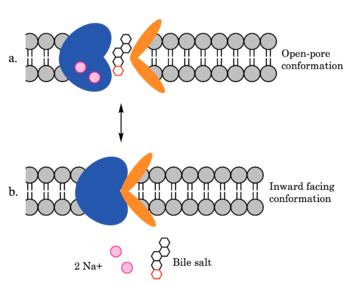
| - | [[Image:ntcpmechanismoverall.png|350 px|right|thumb| Figure 4. Mechanism of bile salt uptake by NTCP. A bile salt and two Na+ ions bind to NTCP in the open pore state. Transition to the inward-facing state allows release of the bile salt and ions into the cytoplasm. Note that this mechanism does not include the second, non-transported | + | [[Image:ntcpmechanismoverall.png|350 px|right|thumb| Figure 4. Mechanism of bile salt uptake by NTCP. A bile salt and two Na+ ions bind to NTCP in the open pore state. Transition to the inward-facing state allows release of the bile salt and ions into the cytoplasm. Note that this mechanism does not include the second, non-transported bile salt indicated in the mechanism.]] |
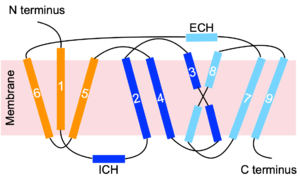
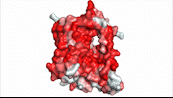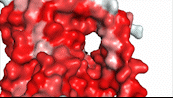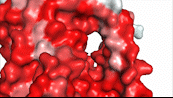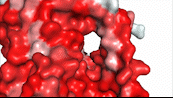
NTCP utilizes secondary active transport to uptake bile salts from blood plasma into the cytoplasm of liver cells (Figure 4). The transport of one bile salt is driven by the downhill transport of 2 Na+ ions along sodium’s electrochemical gradient. Two bile salts and two Na+ ions bind to the <scene name='95/952697/Ntcp_open-pore_state_surface/4'>open pore state</scene> (Figure 4a), but only one bile salt (along with two Na+ ions) is released into the cytoplasm.<ref name="Liu" /> After all substrates are bound, the conformational change from the open pore state to the inward-facing state is driven by the energetics of the favorable transport of Na+ ions.<ref name="Liu" /> The <scene name='95/952697/Ntcp_inward_facing_state/3'>inward-facing state</scene> (Figure 4b) allows release of one bile salt and two Na+ ions into the cytoplasm. In this conformation, the pore closes off relative to the extracellular side and opens to the cytoplasmic side.<ref name="Asami" /> Afterwards, the remaining bile salt bound to NTCP shifts to the position the previous bile salt occupied, and the process repeats itself.<ref name = "Liu" /> The inherent amiphipathicity of bile acids allows passage through NTCP’s amphipathic pore (Figure 5).[[Image:Hydro_NEWEST_AdobeExpress_(1).gif|400 px|right|thumb|Figure 5. Amphipathic pore of NTCP highlighting hydrophobic residues (red) and hydrophilic residues (white). (PDB: [https://www.rcsb.org/structure/7PQQ 7PQQ])]] | NTCP utilizes secondary active transport to uptake bile salts from blood plasma into the cytoplasm of liver cells (Figure 4). The transport of one bile salt is driven by the downhill transport of 2 Na+ ions along sodium’s electrochemical gradient. Two bile salts and two Na+ ions bind to the <scene name='95/952697/Ntcp_open-pore_state_surface/4'>open pore state</scene> (Figure 4a), but only one bile salt (along with two Na+ ions) is released into the cytoplasm.<ref name="Liu" /> After all substrates are bound, the conformational change from the open pore state to the inward-facing state is driven by the energetics of the favorable transport of Na+ ions.<ref name="Liu" /> The <scene name='95/952697/Ntcp_inward_facing_state/3'>inward-facing state</scene> (Figure 4b) allows release of one bile salt and two Na+ ions into the cytoplasm. In this conformation, the pore closes off relative to the extracellular side and opens to the cytoplasmic side.<ref name="Asami" /> Afterwards, the remaining bile salt bound to NTCP shifts to the position the previous bile salt occupied, and the process repeats itself.<ref name = "Liu" /> The inherent amiphipathicity of bile acids allows passage through NTCP’s amphipathic pore (Figure 5).[[Image:Hydro_NEWEST_AdobeExpress_(1).gif|400 px|right|thumb|Figure 5. Amphipathic pore of NTCP highlighting hydrophobic residues (red) and hydrophilic residues (white). (PDB: [https://www.rcsb.org/structure/7PQQ 7PQQ])]] | ||
=== Mechanism of HBV/HDV Infection === | === Mechanism of HBV/HDV Infection === | ||
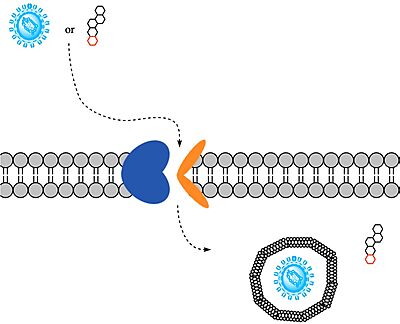
After binding to NTCP in the <scene name='95/952697/Ntcp_open-pore_state/26'>open pore state</scene>, the viruses remain bound until low bile salt levels in the blood shift equilibria enough that [https://en.wikipedia.org/wiki/Endocytosis endocytosis] of the virus occurs. Once inside the cell, the viral genetic information is released. | After binding to NTCP in the <scene name='95/952697/Ntcp_open-pore_state/26'>open pore state</scene>, the viruses remain bound until low bile salt levels in the blood shift equilibria enough that [https://en.wikipedia.org/wiki/Endocytosis endocytosis] of the virus occurs. Once inside the cell, the viral genetic information is released. | ||
| - | + | Although the exact mechanism of how HBV and HDV bind to NTCP is not certain, <scene name='95/952696/Residues_84-87_and_157-165_new/1'>two critical sites</scene> on NTCP for HBV/HDV binding have been identified: residues <scene name='95/952696/Residues_84-87_new/1'>84-87</scene> and <scene name='95/952696/Residues_157-165_new/1'>157-165</scene>. An additional [https://en.wikipedia.org/wiki/Single-nucleotide_polymorphism single-nucleotide polymorphism] was discovered in East Asia involving <scene name='95/952696/Residue_267_new/15'>residue 267</scene> being mutated from serine to phenylalanine.<ref name="Asami" /> This mutation prevented HBV/HDV infection presumably by blocking the binding site in the pore of NTCP. Another mutation, replacing <scene name='95/952696/Leucine_residues/2'>L27, L31, and L35</scene> with tryptophan residues, prevents HBV/HDV infection.<ref name="Park"/> A <scene name='95/952696/Leucine_residues_zoomed/2'>closer view of L27, L31, and L35</scene> in the pore demonstrates how this mutation to bulkier tryptophan residues would block the preS1 binding site of HBV/HDV. [https://en.wikipedia.org/wiki/Myristoylation Myristoylation] of the HBV/HDV capsid is also vital for recognition by NTCP, as well as residues 8-17 on HBV/HDV (sequence: NPLGFFPDHQ)<ref name="Park"/>. Two mechanisms have been proposed for how HBV/HDV binds to NTCP. The first mechanism involves binding of the myristoyl group to the host cell membrane, while residues 8-17 interact with NTCP residues <scene name='95/952696/Residues_157-165_new/1'>157-165</scene>. The second mechanism involves binding of the myristoyl group with residues 157-165 in the pore.<Ref name="Zhang"> Zhang X, Zhang Q, Peng Q, Zhou J, Liao L, Sun X, Zhang L, Gong T. Hepatitis B virus preS1-derived lipopeptide functionalized liposomes for targeting of hepatic cells. Biomaterials. 2014 Jul;35(23):6130-41. [https://dx.doi.org/10.1016/j.biomaterials.2014.04.037 DOI: 10.1016/j.biomaterials.2014.04.037]. </Ref> After successful binding via one of these two proposed mechanisms, the virus is eventually endocytosed. | |
== Medical Relevance == | == Medical Relevance == | ||
Current revision
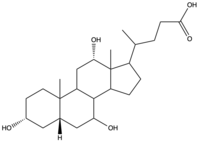
Sodium-taurocholate Co-transporting Polypeptide
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Goutam K, Ielasi FS, Pardon E, Steyaert J, Reyes N. Structural basis of sodium-dependent bile salt uptake into the liver. Nature. 2022 Jun;606(7916):1015-1020. DOI: 10.1038/s41586-022-04723-z.
- ↑ 2.0 2.1 2.2 2.3 2.4 Asami J, Kimura KT, Fujita-Fujiharu Y, Ishida H, Zhang Z, Nomura Y, Liu K, Uemura T, Sato Y, Ono M, Yamamoto M, Noda T, Shigematsu H, Drew D, Iwata S, Shimizu T, Nomura N, Ohto U. Structure of the bile acid transporter and HBV receptor NTCP. Nature. 2022 Jun; 606 (7916):1021-1026. DOI: 10.1038/s41586-022-04845-4.
- ↑ 3.0 3.1 3.2 3.3 Park JH, Iwamoto M, Yun JH, Uchikubo-Kamo T, Son D, Jin Z, Yoshida H, Ohki M, Ishimoto N, Mizutani K, Oshima M, Muramatsu M, Wakita T, Shirouzu M, Liu K, Uemura T, Nomura N, Iwata S, Watashi K, Tame JRH, Nishizawa T, Lee W, Park SY. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature. 2022 Jun;606(7916):1027-1031. DOI: 10.1038/s41586-022-04857-0.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Liu H, Irobalieva RN, Bang-Sørensen R, Nosol K, Mukherjee S, Agrawal P, Stieger B, Kossiakoff AA, Locher KP. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res. 2022 Aug;32(8):773-776. DOI: 10.1038/s41422-022-00680-4.
- ↑ Qi X, Li W. Unlocking the secrets to human NTCP structure. Innovation (Camb). 2022 Aug 1;3(5):100294. DOI: 10.1016/j.xinn.2022.100294.
- ↑ 6.0 6.1 Zhang X, Zhang Q, Peng Q, Zhou J, Liao L, Sun X, Zhang L, Gong T. Hepatitis B virus preS1-derived lipopeptide functionalized liposomes for targeting of hepatic cells. Biomaterials. 2014 Jul;35(23):6130-41. DOI: 10.1016/j.biomaterials.2014.04.037.
- ↑ 7.0 7.1 7.2 Patton JS, Carey MC. Watching fat digestion. Science. 1979 Apr 13;204(4389):145-8. DOI: 10.1126/science.432636.
- ↑ Donkers JM, Kooijman S, Slijepcevic D, Kunst RF, Roscam Abbing RL, Haazen L, de Waart DR, Levels JH, Schoonjans K, Rensen PC, Oude Elferink RP, van de Graaf SF. NTCP deficiency in mice protects against obesity and hepatosteatosis. JCI Insight. 2019 Jun 25;5(14):e127197. DOI: 10.1172/jci.insight.127197.
- ↑ 9.0 9.1 Shimura S, Watashi K, Fukano K, Peel M, Sluder A, Kawai F, Iwamoto M, Tsukuda S, Takeuchi JS, Miyake T, Sugiyama M, Ogasawara Y, Park SY, Tanaka Y, Kusuhara H, Mizokami M, Sureau C, Wakita T. Cyclosporin derivatives inhibit hepatitis B virus entry without interfering with NTCP transporter activity. J Hepatol. 2017 Apr;66(4):685-692. doi: 10.1016/j.jhep.2016.11.009. DOI: 10.1016/j.jhep.2016.11.009.
Student Contributors
- Ben Minor
- Maggie Samm
- Zac Stanley