This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1794
From Proteopedia
(Difference between revisions)
| (15 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
='''Sodium Taurocholate Co-Transporting Polypeptide'''= | ='''Sodium Taurocholate Co-Transporting Polypeptide'''= | ||
| - | <StructureSection load='' size='350' side='right' scene='95/952721/Structure_overview/ | + | <StructureSection load='7zyi' size='350' side='right' caption='Sodium Bile Salt Co-Transporting Polypeptide (NTCP) with green bile salts and yellow sodium ions bound [https://www.rcsb.org/structure/7ZYI PDB: 7zyi]' scene ='95/952721/Structure_overview/8'> |
== Introduction == | == Introduction == | ||
| - | [[image:Taurocholate.png|thumb|250 px| '''Fig. 1: | + | [[image:Taurocholate.png|thumb|250 px| '''Fig.1: Taruocholic acid a crystalline bile acid''' Shown here is the structure in a line representation of taruocholic acid with stereochemistry and important hydrogens.]] |
| - | Sodium Taurocholate Co-Transporting Polypeptide, or NTCP, is a [https://en.wikipedia.org/wiki/Membrane_transport_protein membrane transporter protein] found in the plasma membrane of [https://en.wikipedia.org/wiki/Hepatocyte hepatocytes]. NTCP's primary function is the transportation of [https://en.wikipedia.org/wiki/Taurocholic_acid taurocholates], or '''bile salts''', (Fig. 1) into the liver and out of the liver to the small intestine. <Ref> Stieger B. The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb Exp Pharmacol. 2011;(201):205-59. doi: 10.1007/978-3-642-14541-4_5. PMID: 21103971. [https://dx.doi.org/10.1007/978-3-642-14541-4_5 DOI: DOI: 10.1007/978-3-642-14541-4_5]. </Ref> Bile salts play various roles in metabolism and digestion, but their main function is the [https://en.wikipedia.org/wiki/Emulsion emulsification] of lipid droplets into smaller fragments. This enables lipases to break down the droplets into their monomers, or triglycerides which are then able to be digested. NTCP is part of the [https://en.wikipedia.org/wiki/Solute_carrier_family solute carrier superfamily], SLC10. NTCP is the founding member of the SLC10 family, first discovered in rat hepatocytes in 1978. <ref name = "SLC10"> Geyer, J., Wilke, T. & Petzinger, E. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmied Arch Pharmacol 372, 413–431 (2006). https://doi.org/10.1007/s00210-006-0043-8 </ref> NTCP has a key role in [https://en.wikipedia.org/wiki/Enterohepatic_circulation enterohepatic circulation] or '''bile salt recycling''', and its unique ability to transport other solutes gives it therapeutic potential for lowering cholesterol and treating [https://en.wikipedia.org/wiki/Liver_disease liver disease]. | + | Sodium Taurocholate Co-Transporting Polypeptide, or NTCP, is a [https://en.wikipedia.org/wiki/Membrane_transport_protein membrane transporter protein] found in the plasma membrane of [https://en.wikipedia.org/wiki/Hepatocyte hepatocytes]. NTCP's primary function is the transportation of [https://en.wikipedia.org/wiki/Taurocholic_acid taurocholates], or '''bile salts''', (Fig. 1) into the liver and out of the liver to the small intestine. <Ref> Stieger B. The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb Exp Pharmacol. 2011;(201):205-59. doi: 10.1007/978-3-642-14541-4_5. PMID: 21103971. [https://dx.doi.org/10.1007/978-3-642-14541-4_5 DOI: DOI: 10.1007/978-3-642-14541-4_5]. </Ref> Bile salts play various physiological roles in metabolism and digestion, but their main function is the [https://en.wikipedia.org/wiki/Emulsion emulsification] of lipid droplets into smaller fragments. This enables lipases to break down the droplets into their monomers, or triglycerides which are then able to be digested. NTCP is part of the [https://en.wikipedia.org/wiki/Solute_carrier_family solute carrier superfamily], SLC10. NTCP is the founding member of the SLC10 family, first discovered in rat hepatocytes in 1978. <ref name = "SLC10"> Geyer, J., Wilke, T. & Petzinger, E. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmied Arch Pharmacol 372, 413–431 (2006). https://doi.org/10.1007/s00210-006-0043-8 </ref> NTCP has a key role in [https://en.wikipedia.org/wiki/Enterohepatic_circulation enterohepatic circulation] or '''bile salt recycling''', and its unique ability to transport other solutes gives it therapeutic potential for lowering cholesterol and treating [https://en.wikipedia.org/wiki/Liver_disease liver disease]. <Ref name = "Goutam"/> |
In addition to its physiological role in bile salt transport, NTCP also serves as a binding site for [https://en.wikipedia.org/wiki/Hepatitis_B hepatitis B virus] and [https://en.wikipedia.org/wiki/Hepatitis_D hepatitis D virus]. <ref name = "Park"> Park, JH., Iwamoto, M., Yun, JH. et al. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature 606, 1027–1031 (2022). https://doi.org/10.1038/s41586-022-04857-0. </ref> Understanding the HBV and HDV binding mechanism to NTCP may aid in the development of new viral inhibitors. | In addition to its physiological role in bile salt transport, NTCP also serves as a binding site for [https://en.wikipedia.org/wiki/Hepatitis_B hepatitis B virus] and [https://en.wikipedia.org/wiki/Hepatitis_D hepatitis D virus]. <ref name = "Park"> Park, JH., Iwamoto, M., Yun, JH. et al. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature 606, 1027–1031 (2022). https://doi.org/10.1038/s41586-022-04857-0. </ref> Understanding the HBV and HDV binding mechanism to NTCP may aid in the development of new viral inhibitors. | ||
| Line 14: | Line 14: | ||
=== Overview === | === Overview === | ||
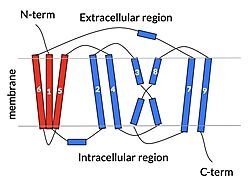
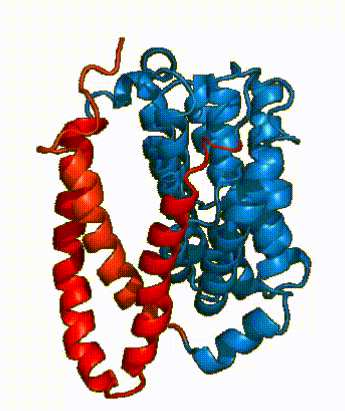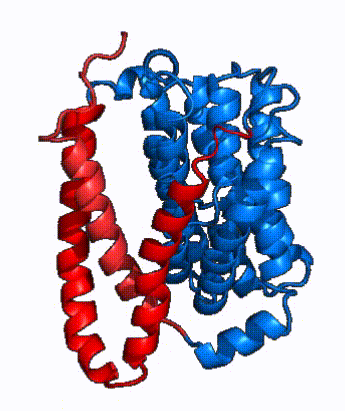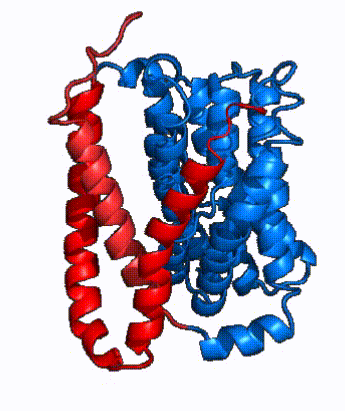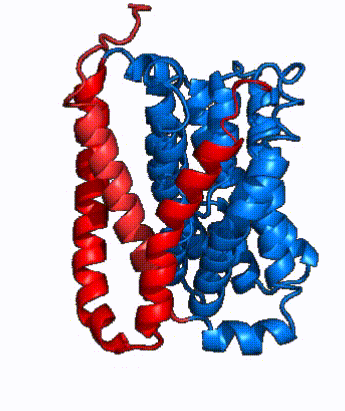
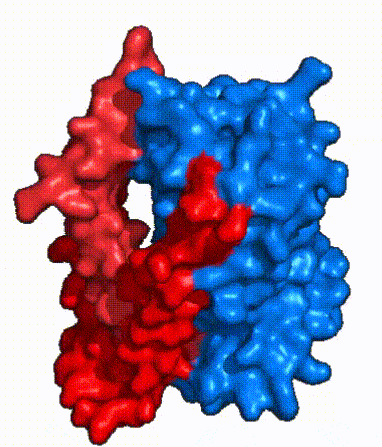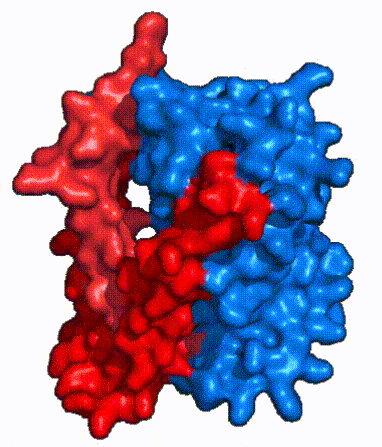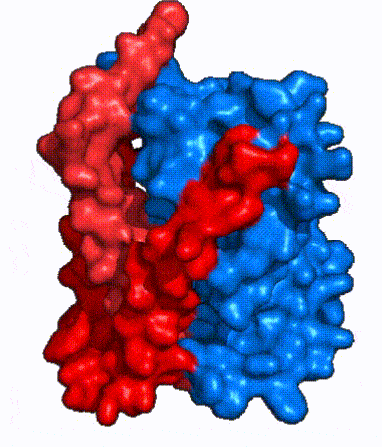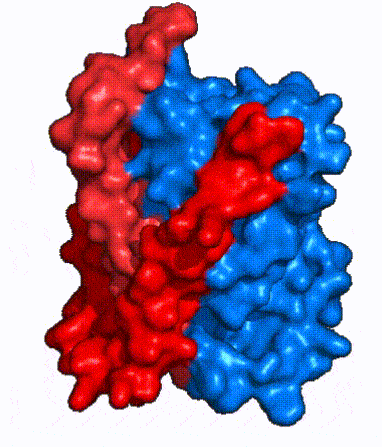
| - | NTCP is one continuous polypeptide chain containing <scene name='95/952722/Labeled_9_helices/5'>9 transmembrane alpha helices</scene>.<ref name="Goutam"/> The N-terminus of | + | The overall structure of NTCP is one continuous polypeptide chain containing <scene name='95/952722/Labeled_9_helices/5'>9 transmembrane alpha helices</scene> embedded in the plasma membrane.<ref name="Goutam"/> The N-terminus of this polypeptide chain extrudes into the extracellular region of the plasma membrane while the C-terminus juts into the intracellular region. NTCP contains <scene name='95/952722/Ntcp_core_domain-_blue/10'>two distinct sub domains</scene>: a core domain and a panel domain, which together channel opening and bile salt transport (Fig. 2). The <scene name='95/952722/Ntcp_core_domain-_blue/8'>core domain</scene> <font color='#6060ff'><b>(blue)</b></font> contains 6 transmembrane α helices (TM2-4 and TM7-9) and demonstrates [https://en.wikipedia.org/wiki/Protein_structure two-fold pseudosymmetry]. The <scene name='95/952722/Ntcp_panel_domain-_red/4'>panel domain</scene> <font color='red'><b>(red)</b></font> consists of 3 transmembrane α helices (TM1 and TM5-6) and is asymmetrical. Within the core domain, a unique crossover between TM-3 and TM-8 creates an <scene name='95/952722/Ntcp_x_motif/14'>X motif</scene>. The X motif contains the substrate binding site and essential residues for the conformational change required for transport. The core and panel domains are also connected by both extracellular and intracellular <scene name='95/952722/Connector_helices/6'>connector helices</scene> that are separate from the <scene name='95/952722/Labeled_9_helices/5'>9 transmembrane alpha helices</scene>. All of these structural components of NTCP contribute to the transport of bile salts in and out of the liver. |
| Line 20: | Line 20: | ||
==== Sodium ==== | ==== Sodium ==== | ||
| - | NTCP, | + | One of NTCP's key structural features, like other SLC10 family members, is <scene name='95/952721/Sodium_binding/5'>two sodium binding sites</scene>. Many polar and negatively charged residues (68, 105, 106, 119, 123, 257, 261) form ion-dipole or dipole-dipole interactions with the sodium ions in these sites with a high level of conservation, suggesting sodium binding is coupled to bile salt transport. <Ref name = "Goutam"/> Mutations in the X-motif near sodium binding sites also inhibit bile salt transport function, suggesting that sodium is required for salt binding. |
| - | <Ref name = "Goutam"> Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). [https://doi.org/10.1038/s41586-022-04723-z DOI: 10.1038/s41586-022-04723-z]. </Ref> | + | <Ref name = "Goutam"> Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). [https://doi.org/10.1038/s41586-022-04723-z DOI: 10.1038/s41586-022-04723-z]. </Ref> Sodium transport facilitates structural changes in NTCP from its typical open-pore state to an inward-facing (closed-pore) state. The inward-facing state is favored in the absence of sodium ions, while open-pore state is favored in the presence of sodium ions. <Ref name = "Goutam"/> Gating of the channel with sodium in this way allows for sodium concentrations to regulate uptake of taurocholates. <Ref name = "Goutam"/> When intracellular sodium levels are higher, open-pore state is favored allowing for the diffusion of taurocholates. However, when extracellular sodium levels are high, inward-facing state is favored preventing diffusion of taurocholates. <ref name="Goutam"/> Overall, this suggests that thermodynamically favorable sodium transport is coupled to moving bile salts against their concentration gradient. <Ref name = "Liu"/> |
==== Bile Salts ==== | ==== Bile Salts ==== | ||
| - | + | Another key feature of NTCP is its <scene name='95/952722/Amphipathic_patterns/1'>amphipathic pore</scene> which allows for bile salt transport across the hydrophilic membrane. The pore surface remains {{Template:ColorKey_Hydrophobic}}, while the <scene name='95/952721/Amphipathic_patterns_pore/2'>lining of the open pore</scene> is largely {{Template:ColorKey_Polar}}. In the inward-facing or closed-pore conformation, the polar pore residues are inaccessible. Only the surface hydrophobic residues are exposed. As the pore opens up, inner polar residues become accessible allowing for the binding of hydrophilic bile salts. The pattern of hydrophobic and polar residues within the pore matches the amphipathic patterns within taurocholates, [https://en.wikipedia.org/wiki/Steroid steroids], and [https://en.wikipedia.org/wiki/Thyroid_hormones thyroid hormones]. <Ref name = Qi> Qi X. and Li W. (2022). Unlocking the secrets to human NTCP structure. The Innovation 3(5), 100294. https://doi.org/10.1016/j.xinn.2022.100294 </ref> Using this amphipathic pore, provides the channel with specificity while preventing leakage of other substrates. Essential <scene name='95/952722/Bile_salts_res/1'>bile salt binding residues</scene> form Van der Waals interactions with bile salt substrates, while others form dipole-dipole or ionic interactions. The core domain contributes most of the polar domains, while the panel domain contributes mainly hydrophobic surface. | |
=== Conformational Change === | === Conformational Change === | ||
| Line 33: | Line 33: | ||
| [[Image:Surface_NTCP_morph.gif]] | | [[Image:Surface_NTCP_morph.gif]] | ||
|- | |- | ||
| - | | '''Fig. 3: NTCP shown as cartoons with <font color='red'><b>panel</b></font> and <font color='#6060ff'><b>core</b></font> domains colored.''' | + | | '''Fig. 3: NTCP shown as cartoons with <font color='red'><b>panel</b></font> and <font color='#6060ff'><b>core</b></font> domains colored.''' NTCP is shown entirely as cartoons colored by domain. The panel domain helices can be seen moving to close the pore. Here NTCP is rotated 180° from how it is normally oriented and is alternating from open-pore to inward-facing conformation (7PQQ to 7PQG). |
| - | | '''Fig. 4: NTCP surface representation with <font color='red'><b>panel</b></font> and <font color='#6060ff'><b>core</b></font> domains colored.''' | + | | '''Fig. 4: NTCP surface representation with <font color='red'><b>panel</b></font> and <font color='#6060ff'><b>core</b></font> domains colored.''' NTCP is shown as surface representation colored by domain. The panel domain can be seen shifting to close the pore. Here NTCP is rotated 180° from how it is normally oriented and is alternating from open-pore to inward-facing conformation (7PQQ to 7PQG). |
|} | |} | ||
| - | In order to reveal these binding sites to initiate bile salt transport, NTCP exists in two different conformations; the <scene name='95/952722/Open_pore_conf/4'>open pore conformation</scene> and the <scene name='95/952722/Inward_facing_conf/1'>inward facing conformation</scene>. <ref name="Goutam"/> NTCP undergoes a conformational change from inward facing to open pore which exposes the binding sites to the extracellular region to allow the sodium ions and bile salts to bind. In this movement, the <scene name='95/952722/Ntcp_core_domain-_blue/8'>core domain</scene> and the <scene name='95/952722/Ntcp_panel_domain-_red/4'>panel domain</scene> rotate 20° with the <font color='red'><b>panel domain</b></font> moving 5 Å away from the <font color='#6060ff'><b>core domain</b></font>, which remains relatively rigid. This conformational change reveals the two sodium ion binding sites as well as the <scene name='95/952721/Amphipathic_patterns/2'>amphipathic pore</scene> in the membrane. The movement of the panel domain is facilitated by <scene name='95/952722/Pro_and_gly_hinges/5'>proline and glycine residues</scene> located in the <scene name='95/952722/Connector_helices/6'>connector helices</scene> between the panel and core domains. <scene name='95/952722/Pro_and_gly_hinges/7'>These residues</scene> <font color='#FCE205'><b>(yellow)</b></font> act as hinges that assist in the movement of the panel domain away from the core domain. <ref name="Goutam"/> | + | In order to reveal these binding sites to initiate bile salt transport, NTCP exists in two different conformations; the <scene name='95/952722/Open_pore_conf/4'>open pore conformation</scene> and the <scene name='95/952722/Inward_facing_conf/1'>inward facing conformation</scene>. <ref name="Goutam"/> NTCP undergoes a conformational change from inward facing to open pore which exposes the binding sites to the extracellular region to allow the sodium ions and bile salts to bind. NTCP utilizes an [https://www.sciencedirect.com/science/article/pii/S0092867417302891 elevator-alternating mechanism] <Ref name = "Latorraca"> Latorraca, N. R.; Fastman, N. M.; Venkatakrishnan, A. J.; Frommer, W. B.; Dror, R. O.; Feng, L. Mechanism of Substrate Translocation in an Alternating Access Transporter. Cell 2017, 169 (1), 96–107. </ref> where one domain <font color='red'><b>(panel)</b></font> does most of the translocation, and the other domain <font color='#6060ff'><b>(core)</b></font> remains stationary. <Ref name = "Asami"> Asami, J., Kimura, K.T., Fujita-Fujiharu, Y. et al.Structure of the bile acid transporter and HBV receptor NTCP. Nature 606, 1021–1026 (2022). https://doi.org/10.1038/s41586-022-04845-4 </ref> In this movement, the <scene name='95/952722/Ntcp_core_domain-_blue/8'>core domain</scene> and the <scene name='95/952722/Ntcp_panel_domain-_red/4'>panel domain</scene> rotate 20° with the <font color='red'><b>panel domain</b></font> moving 5 Å away from the <font color='#6060ff'><b>core domain</b></font>, which remains relatively rigid. This conformational change reveals the two sodium ion binding sites as well as the <scene name='95/952721/Amphipathic_patterns/2'>amphipathic pore</scene> in the membrane. The movement of the panel domain is facilitated by <scene name='95/952722/Pro_and_gly_hinges/5'>proline and glycine residues</scene> located in the <scene name='95/952722/Connector_helices/6'>connector helices</scene> between the panel and core domains. <scene name='95/952722/Pro_and_gly_hinges/7'>These residues</scene> <font color='#FCE205'><b>(yellow)</b></font> act as hinges that assist in the movement of the panel domain away from the core domain. <ref name="Goutam"/> |
== Bile Salt Transport == | == Bile Salt Transport == | ||
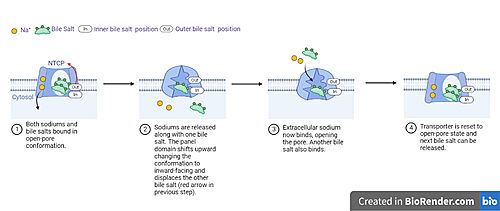
| - | [[image: | + | [[image: NTCP_transport.jpg|left|thumb|500 px| '''Fig. 5 Proposed process of NTCP bile salt transport.''' NTCP structure is shown in light blue with a white channel going through the protein. Sodium ions as yellow spheres and bile salts as green blobs with inner and outer binding sites labeled. Captions below each image detail how movement leads to transport of bile salts and conformational changes in NTCP throughout the different steps of the process.]] |
| - | A proposed pathway for NTCP bile salt transport | + | A proposed pathway for NTCP bile salt transport starting and ending with open-pore states hypothesizes that both sodium ions are translocated with the transport of one bile salt.<Ref name = "Liu"> Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). https://doi.org/10.1038/s41422-022-00680-4 </Ref>. Only one taurocholate is transported at a time due to NTCP's two bile salt binding sites. An |
| + | <scene name='95/952721/Inner_bile_salt/2'>inner bile salt</scene> that is closer to the cytoplasmic side of the membrane and an <scene name='95/952721/Outer_bile_salt/2'>outer bile salt</scene> that is closer to the extracellular side of the membrane <Ref name = "Liu"/> In the open-pore state both <scene name='95/952721/Mech_step_1/1'>taurocholates and sodium ions bound.</scene> Next both sodium ions are released into the cytoplasm along with the inner bile salt into the cytoplasm (Fig. 5). Once the sodium ions are released this will trigger the conformational change in NTCP. The <scene name='95/952721/Mech_step_2/2'>outermost bile salt remains bound</scene> in the pore, likely helping to prevent leakage. <Ref name = "Liu"/> Then the <scene name='95/952722/Mech_step_3/1'>outer bile salt is displaced</scene> into the inner bile salt binding site by the movement from conformational change to the inward-facing, pore inaccessible state due to sodium ion release (Fig. 5). <Ref name = "Liu"/> Two new sodium ions then bind to NTCP from the extracellular side, favoring the open-pore state and also allowing for the binding of another outer bile salt (Fig 5). The <scene name='95/952721/Mech_step_1/1'>protein is then reset</scene> and the process can then start again releasing the next inner bile salt with the translocation of the sodium ions into the cytoplasm. This mechanism melds typical patterns of [https://proteopedia.org/wiki/index.php/Pump gated channels and pumps] in a new light as sodium ions control conformation and thus binding, displacement, and release of bile salts. <Ref name = "Goutam"/> | ||
== HBV Binding and Infection== | == HBV Binding and Infection== | ||
| - | NTCP is the only [https://rupress.org/jcb/article/195/7/1071/54877/The-cell-biology-of-receptor-mediated-virus entry receptor] <Ref name = "Grove"> Grove, J.; Marsh, M. The Cell Biology of Receptor-Mediated Virus Entry. Journal of Cell Biology 2011, 195 (7), 1071–1082. </ref> into the liver for HBV. <Ref name = "Asami"/> The [https://en.wikipedia.org/wiki/Myristoylation myristolated] PreS1 domain of HBV binds to NTCP through | + | The other main function of NTCP is its role as the only [https://rupress.org/jcb/article/195/7/1071/54877/The-cell-biology-of-receptor-mediated-virus entry receptor] <Ref name = "Grove"> Grove, J.; Marsh, M. The Cell Biology of Receptor-Mediated Virus Entry. Journal of Cell Biology 2011, 195 (7), 1071–1082. </ref> into the liver for HBV and HDV. <Ref name = "Asami"/>These viruses are known to use <scene name='95/952721/Hep_patches/2'>two different patches</scene> <font color='#00e080'><b>(residues 84-87 and 157-165)</b></font> on NTCP for binding and entry. The [https://en.wikipedia.org/wiki/Myristoylation myristolated] PreS1 domain of HBV binds to NTCP through the <scene name='95/952721/Hbv_patch_1/1'>first hydrophobic patch</scene> on NTCP containing <font color='#00e080'><b>residues 157-165</b></font> on the open pore surface. <Ref name = "Asami"/> These residues form part of the bile salt transport tunnel resulting in HBV binding and bile salt transport directly competing and interfering with one another. <Ref name = "Asami"/> The <scene name='95/952721/Hbv_patch_2/1'>other hydrophobic patch</scene> consisting of <font color='#00e080'><b>residues 84-87</b></font> found on the N-terminus of NTCP does not overlap with bile salt binding and may be used for the development of [https://en.wikipedia.org/wiki/Antiviral_drug antivirals] that do not inhibit bile uptake <Ref name = "Park"/>. Other minor variations within NTCP provide species specificity for HBV or virus resistance, such as mutant S267F found in East Asia. <Ref name = "Park"/> This S267F mutation is a [https://en.wikipedia.org/wiki/Single-nucleotide_polymorphism single-nucleotide polymorphism], where a change in one nucleotide in the sequence has caused a lack of bile salt transport activity or viral infection. <Ref name = "Park"/> It is hypothesized that due to the lack of bile salt transport in this mutation that the open-pore state during bile salt transport is necessary for HBV and HDV infection, suggesting the two functionally overlap. <Ref name = "Park"/> |
| - | The exact mechanism by which NTCP mediates viral internalization is still | + | The exact mechanism by which NTCP mediates viral internalization is still being determined; however, current evidence suggests it works through [https://en.wikipedia.org/wiki/Viral_entry#Entry_via_endocytosis endocytosis.] <Ref name = "Herrscher"> Herrscher C, Roingeard P, Blanchard E. Hepatitis B Virus Entry into Cells. Cells. 2020 Jun 18;9(6):1486. doi: 10.3390/cells9061486. PMID: 32570893; PMCID: PMC7349259. </ref> Once HBV is bound, the NTCP/HBV complex is taken into the cell where viral contents are dumped into the cytoplasm to then begin [https://en.wikipedia.org/wiki/Viral_replication viral replication]. HBV may also interact with other receptors or host cell factors, as cells overexpressing NTCP alone had low infection efficiency. <Ref name = "Herrscher"/> |
| Line 54: | Line 55: | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | |||
| + | == PDB Files == | ||
| + | *Bile salts and sodium ions bound: [https://www.rcsb.org/structure/7ZYI 7zyi] | ||
| + | *Open-pore NTCP: [https://www.rcsb.org/structure/7PQQ 7pqq] | ||
| + | *Inward-facing NTCP: [https://www.rcsb.org/structure/7PQG 7pqg] | ||
== Student Contributors == | == Student Contributors == | ||
Current revision
Contents |
Sodium Taurocholate Co-Transporting Polypeptide
| |||||||||||
References
- ↑ Stieger B. The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb Exp Pharmacol. 2011;(201):205-59. doi: 10.1007/978-3-642-14541-4_5. PMID: 21103971. DOI: DOI: 10.1007/978-3-642-14541-4_5.
- ↑ Geyer, J., Wilke, T. & Petzinger, E. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmied Arch Pharmacol 372, 413–431 (2006). https://doi.org/10.1007/s00210-006-0043-8
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). DOI: 10.1038/s41586-022-04723-z.
- ↑ 4.0 4.1 4.2 4.3 4.4 Park, JH., Iwamoto, M., Yun, JH. et al. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature 606, 1027–1031 (2022). https://doi.org/10.1038/s41586-022-04857-0.
- ↑ 5.0 5.1 5.2 5.3 5.4 Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). https://doi.org/10.1038/s41422-022-00680-4
- ↑ Qi X. and Li W. (2022). Unlocking the secrets to human NTCP structure. The Innovation 3(5), 100294. https://doi.org/10.1016/j.xinn.2022.100294
- ↑ Latorraca, N. R.; Fastman, N. M.; Venkatakrishnan, A. J.; Frommer, W. B.; Dror, R. O.; Feng, L. Mechanism of Substrate Translocation in an Alternating Access Transporter. Cell 2017, 169 (1), 96–107.
- ↑ 8.0 8.1 8.2 8.3 Asami, J., Kimura, K.T., Fujita-Fujiharu, Y. et al.Structure of the bile acid transporter and HBV receptor NTCP. Nature 606, 1021–1026 (2022). https://doi.org/10.1038/s41586-022-04845-4
- ↑ Grove, J.; Marsh, M. The Cell Biology of Receptor-Mediated Virus Entry. Journal of Cell Biology 2011, 195 (7), 1071–1082.
- ↑ 10.0 10.1 Herrscher C, Roingeard P, Blanchard E. Hepatitis B Virus Entry into Cells. Cells. 2020 Jun 18;9(6):1486. doi: 10.3390/cells9061486. PMID: 32570893; PMCID: PMC7349259.
PDB Files
Student Contributors
- Isabelle White
- Lena Barko