Sandbox Reserved 1777
From Proteopedia
(Difference between revisions)
| (10 intermediate revisions not shown.) | |||
| Line 7: | Line 7: | ||
[[Image:Screen Shot 2023-04-16 at 7.30.09 PM.jpeg|650px|thumb|<font size="2"><div style="text-align: center;">'''Figure 1'''. Schematic representation of RAS/RAF/MEK/ERK pathway after assembly of SHOC2-PP1C-MRAS complex. </div></font>]] | [[Image:Screen Shot 2023-04-16 at 7.30.09 PM.jpeg|650px|thumb|<font size="2"><div style="text-align: center;">'''Figure 1'''. Schematic representation of RAS/RAF/MEK/ERK pathway after assembly of SHOC2-PP1C-MRAS complex. </div></font>]] | ||
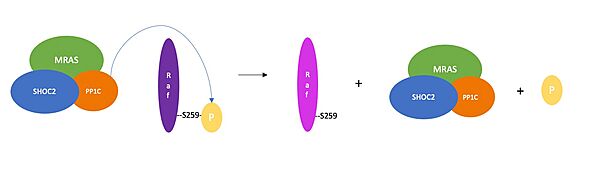
| - | After activation via an extracellular growth factor, the RAS-GTPase enzyme binds GTP, which recruits RAF to the cell membrane. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6311149/#:~:text=Full%20activation%20of%20Raf%20requires,plasma%20membrane%20(Roy%20et%20al RAF ]<Ref name='Molina'>Molina JR, Adjei AA. The Ras/Raf/MAPK pathway. J Thorac Oncol. 2006 Jan;1(1):7-9. [https://doi.org/10.1016/S1556-0864(15)31506-9. DOI:10.1016/S1556-0864(15)31506-9]. </Ref> | + | After activation via an extracellular growth factor, the RAS-GTPase enzyme binds GTP, which recruits RAF to the cell membrane. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6311149/#:~:text=Full%20activation%20of%20Raf%20requires,plasma%20membrane%20(Roy%20et%20al RAF ]is a kinase that stimulates a signaling cascade by phosphorylation of MAPK (also known as MEK in mammals), which activates downstream proteins such as ERK1 and ERK2<Ref name='Molina'>Molina JR, Adjei AA. The Ras/Raf/MAPK pathway. J Thorac Oncol. 2006 Jan;1(1):7-9. [https://doi.org/10.1016/S1556-0864(15)31506-9. DOI:10.1016/S1556-0864(15)31506-9]. </Ref>. ERK1 and ERK2 subsequently activate nuclear transcription factors and kinases, as seen in Figure 1. The RAS/RAF/MEK/ERK pathway is a critical signaling cascade for activating transcription factors and regulating gene expression<Ref name='Li'>Li, L., Zhao, G. D., Shi, Z. et. al.The Ras/Raf/MEK/ERK signaling pathway (Figure 1) and its role in the occurrence and development of HCC. Oncology letters, 12(5), 3045–3050. [https://doi.org/10.3892/ol.2016.5110. DOI:10.3892/ol.2016.5110]. </Ref>. |
| Line 16: | Line 16: | ||
==SHOC2== | ==SHOC2== | ||
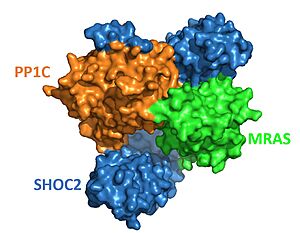
| - | SHOC2 is a scaffolding protein which acts as a cradle to bind PP1C and MRAS <ref name="Hauseman" />, serving as an aggregation point to enable reciprocal interactions between these three signaling proteins. <scene name='95/952705/Shoc2_structure/2'>SHOC2</scene> is a leucine rich repeat ([https://en.wikipedia.org/wiki/Leucine-rich_repeat LRR]) protein consisting of 20 consecutive <scene name='95/952706/Shoc2_structure/9'>LRR motifs</scene> containing <scene name='95/952705/Shoc2_structure/3'>leucine residues</scene>. LRR motifs form an extended β-sheet on the inner concave surface of SHOC2 with α-helices facing outward to facilitate binding of the protein complex. These LRR motifs result in a largely hydrophobic core within the concave region | + | SHOC2 is a scaffolding protein which acts as a cradle to bind PP1C and MRAS <ref name="Hauseman" />, serving as an aggregation point to enable reciprocal interactions between these three signaling proteins. <scene name='95/952705/Shoc2_structure/2'>SHOC2</scene> is a leucine rich repeat ([https://en.wikipedia.org/wiki/Leucine-rich_repeat LRR]) protein consisting of 20 consecutive <scene name='95/952706/Shoc2_structure/9'>LRR motifs</scene> containing <scene name='95/952705/Shoc2_structure/3'>leucine residues</scene>. LRR motifs form an extended β-sheet on the inner concave surface of SHOC2 with α-helices facing outward to facilitate binding of the protein complex. These LRR motifs result in a largely hydrophobic core within the concave region<ref name="Hahn" />. |
==PP1C== | ==PP1C== | ||
| Line 22: | Line 22: | ||
==MRAS== | ==MRAS== | ||
| - | <scene name='95/952705/Mras_structure/4'>MRAS</scene> is a monomeric GTPase and is anchored in the cell membrane by a C-terminal S-Farnesyl cysteine carboxylmethyl ester <Ref name='Zhou'> Zhou, Y., Prakash, P., Liang, H., et al. Lipid-Sorting Specificity Encoded in K-Ras Membrane Anchor Regulates Signal Output. Cell, 168(1-2), 239–251.e16 doi: 10.1016/j.cell.2016.11.059. [https://doi.org/10.1016/j.cell.2016.11.059. DOI: 10.1016/j.cell.2016.11.059]. </Ref>. When MRAS binds GTP, it becomes active, which allows MRAS to bind the rest of the complex<ref name="Hauseman" />. MRAS is a subvariant of the RAS protein and therefore shares most of its regulatory and effector interactions<Ref name= 'Young'>Young, L., Rodriguez-Viciana, P. MRAS: A Close but Understudied Member of the RAS Family. Cold Spring Harbor Perspectives in Medicine (2018). doi: 10.1101/cshperspect.a033621. [https://perspectivesinmedicine.cshlp.org/content/8/12/a033621.full.pdf+html. DOI: 0.1101/cshperspect.a033621]. </Ref>. While other RAS variants bind in complex with SHOC2 and | + | <scene name='95/952705/Mras_structure/4'>MRAS</scene> is a monomeric GTPase and is anchored in the cell membrane by a C-terminal S-Farnesyl cysteine carboxylmethyl ester <Ref name='Zhou'> Zhou, Y., Prakash, P., Liang, H., et al. Lipid-Sorting Specificity Encoded in K-Ras Membrane Anchor Regulates Signal Output. Cell, 168(1-2), 239–251.e16 doi: 10.1016/j.cell.2016.11.059. [https://doi.org/10.1016/j.cell.2016.11.059. DOI: 10.1016/j.cell.2016.11.059]. </Ref>. When MRAS binds GTP, it becomes active, which allows MRAS to bind the rest of the complex<ref name="Hauseman" />. MRAS is a subvariant of the RAS protein and therefore shares most of its regulatory and effector interactions<Ref name= 'Young'>Young, L., Rodriguez-Viciana, P. MRAS: A Close but Understudied Member of the RAS Family. Cold Spring Harbor Perspectives in Medicine (2018). doi: 10.1101/cshperspect.a033621. [https://perspectivesinmedicine.cshlp.org/content/8/12/a033621.full.pdf+html. DOI: 0.1101/cshperspect.a033621]. </Ref>. While other RAS variants bind in complex with SHOC2 and PP1C to allow it to have phosphatase activity, MRAS binds the tightest. |
===Switch I and II=== | ===Switch I and II=== | ||
| Line 30: | Line 30: | ||
==Stabilizing Interactions in Ternary Complex== | ==Stabilizing Interactions in Ternary Complex== | ||
| - | Once an initial signal to begin complex formation has been received, SHOC2 binds PP1C to form a binary complex. The SHOC2-PP1C complex then binds to the membrane bound MRAS. <scene name='95/ | + | Once an initial signal to begin complex formation has been received, SHOC2 binds PP1C to form a binary complex. The SHOC2-PP1C complex then binds to the membrane bound MRAS. <scene name='95/952705/Shoc2_pp1c_interaction/3'>PP1C binds</scene> with the ascending loops of the SHOC2 LRR regions, and is further engaged through the N-terminal region of SHOC2 which contains the <scene name='95/952706/Shoc2_rvxf/3'>RVxF motif</scene> <Ref name= 'Jajian'>Kwon, J., Jajian, B., Bian, Y. et al. Comprehensive structure-function evaluation of the SHOC2 holophosphatase reveals disease mechanisms and therapeutic opportunities. In: Proceedings of the American Association for Cancer Research Annual Meeting 2022. [https://aacrjournals.org/cancerres/article/82/12_Supplement/LB029/699443. DOI: 10.1158/1538-7445.AM2022-LB029]. </Ref>. The initial forming of the complex begins with SHOC2-PP1C engagement, then is completed and stabilized by the GTP-loaded MRAS binding<Ref name="Jajian" />. <scene name='95/952705/Shoc2_mras_interaction/3'>MRAS binds to SHOC2</scene> exclusively through its concave LRRs<Ref name='Kwan'>Kwon, J.J., Hajian, B., Bian, Y. et al. Structure–function analysis of the SHOC2–MRAS–PP1C holophosphatase complex. Nature 609, 408–415 (2022).doi: 10.1038/s41586-022-04928-2. [https://doi.org/10.1038/s41586-022-04928-2. DOI:10.1038/s41586-022-04928-2] </Ref>, primarily by the descending loop and strands of LRR domains 2-10. Once associated with SHOC2, <scene name='95/952705/Mras_pp1c_interaction/3'>MRAS binds to PP1C</scene>. Binding to MRAS localizes the other two proteins to the RAS signaling regions of the membrane to begin cellular signaling<ref name="Kwan" />. |
==Active Site of PP1C== | ==Active Site of PP1C== | ||
| Line 37: | Line 37: | ||
Notably, NTpS can bind to the PP1C active site without PP1C being in complex with SHOC2 and MRAS. This occurs when RAS binds to RAF, which exposes NTpS on RAF. However, for this reaction, the catalytic activity of PP1C outside of the complex is less efficient. It's hypothesized this is because hydrophobic residues directly C-terminal to the phosphorylated serine bind to the hydrophobic patch of PP1C as well as the hydrophobic SHOC2 C-terminus<ref name="Liau">PMID:35768504</ref>. | Notably, NTpS can bind to the PP1C active site without PP1C being in complex with SHOC2 and MRAS. This occurs when RAS binds to RAF, which exposes NTpS on RAF. However, for this reaction, the catalytic activity of PP1C outside of the complex is less efficient. It's hypothesized this is because hydrophobic residues directly C-terminal to the phosphorylated serine bind to the hydrophobic patch of PP1C as well as the hydrophobic SHOC2 C-terminus<ref name="Liau">PMID:35768504</ref>. | ||
| - | The conserved hydrophobic groove at the SHOC2 C-terminus and the hydrophobic region next to the PP1C active site neighbor each other in the structure of the complex. As a result, the hydrophobic residues downstream from NTpS bind to the hydrophobic patch of PP1C next to the active site and to the hydrophobic groove in the SHOC2 C-terminus. There is still some uncertainty as to how the substrate selectivity works, but these regions could play an essential role in the promiscuous binding of PP1C | + | The conserved hydrophobic groove at the SHOC2 C-terminus and the hydrophobic region next to the PP1C active site neighbor each other in the structure of the complex. As a result, the hydrophobic residues downstream from NTpS bind to the hydrophobic patch of PP1C next to the active site and to the hydrophobic groove in the SHOC2 C-terminus. There is still some uncertainty as to how the substrate selectivity works, but these regions could play an essential role in the promiscuous binding of PP1C<ref name="Liau">PMID:35768504</ref>. |
=Activation of RAF= | =Activation of RAF= | ||
| Line 45: | Line 45: | ||
=Implications= | =Implications= | ||
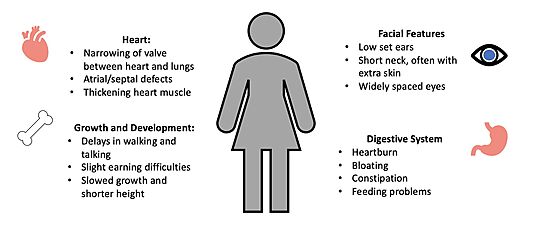
| - | [[Image:Noonan syndrome figure.jpg| | + | [[Image:Noonan syndrome figure.jpg|550px|right|thumb|<font size="2"><div style="text-align: center;">'''Figure 4'''. Common Symptoms of Noonan Syndrome. </div></font>]] |
| - | The SHOC2 | + | The SHOC2-PP1C-MRAS complex's key role in the regulation of the MAPK-RAF pathway means that minor changes in its structure or function can have drastic biological consequences. Unregulated activation of the MAPK pathway is the cause of several human cancers due to unchecked cell division and proliferation<ref name="Kwan" />. Mutations that stabilize the interactions of the SMP complex enhance PP1C phosphatase activity <Ref name="Jajian" />, leading to increased RAF signaling and accelerated cell division. Most SHOC2 or PP1C mutations alter residues that are the direct contact points, stabilizing the interaction between the two proteins. Mutations to MRAS result in persistent binding of GTP, leading to consistent activation of the cell signaling pathway<ref name="Kwan" />. |
The unregulated MAPK pathway is also responsible for a multitude of developmental disorders commonly known as [https://dceg.cancer.gov/research/what-we-study/rasopathies#:~:text=RASopathies%20are%20a%20group%20of,to%20grow%20and%20work%20properly. RASopathies] <Ref name="Lavoie" />. One RASopathy caused by the mutation of a RAF kinase is known as [https://www.mayoclinic.org/diseases-conditions/noonan-syndrome/symptoms-causes/syc-20354422 Noonan Syndrome] (NS), which enhances complex formation by stabilizing the interactions of each member <ref name="Kwan" />. NS is a genetic disorder that can prevent normal development during the neonatal period, leading to difficulties with feeding and a failure to thrive<Ref name= 'van der Burgt'> van der Burgt, I. Noonan syndrome. Orphanet J Rare Dis 2, 4 (2007). doi: 10.1186/1750-1172-2-4 [https://doi.org/10.1186/1750-1172-2-4. DOI: 10.1186/1750-1172-2-4]. </Ref>. The characteristic features of NS become more evident during infancy and childhood. NS patients often have atypical facial appearance, short stature, heart defects, and other physical problems (Figure 4)<ref name="van der Burgt" />. | The unregulated MAPK pathway is also responsible for a multitude of developmental disorders commonly known as [https://dceg.cancer.gov/research/what-we-study/rasopathies#:~:text=RASopathies%20are%20a%20group%20of,to%20grow%20and%20work%20properly. RASopathies] <Ref name="Lavoie" />. One RASopathy caused by the mutation of a RAF kinase is known as [https://www.mayoclinic.org/diseases-conditions/noonan-syndrome/symptoms-causes/syc-20354422 Noonan Syndrome] (NS), which enhances complex formation by stabilizing the interactions of each member <ref name="Kwan" />. NS is a genetic disorder that can prevent normal development during the neonatal period, leading to difficulties with feeding and a failure to thrive<Ref name= 'van der Burgt'> van der Burgt, I. Noonan syndrome. Orphanet J Rare Dis 2, 4 (2007). doi: 10.1186/1750-1172-2-4 [https://doi.org/10.1186/1750-1172-2-4. DOI: 10.1186/1750-1172-2-4]. </Ref>. The characteristic features of NS become more evident during infancy and childhood. NS patients often have atypical facial appearance, short stature, heart defects, and other physical problems (Figure 4)<ref name="van der Burgt" />. | ||
Current revision
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ 1.0 1.1 Bernal Astrain G, Nikolova M, Smith MJ. Functional diversity in the RAS subfamily of small GTPases. Biochem Soc Trans. 2022 Apr 29;50(2):921-933. doi: 10.1042/BST20211166. DOI:10.1042/BST20211166
- ↑ 2.0 2.1 Molina JR, Adjei AA. The Ras/Raf/MAPK pathway. J Thorac Oncol. 2006 Jan;1(1):7-9. DOI:10.1016/S1556-0864(15)31506-9.
- ↑ Li, L., Zhao, G. D., Shi, Z. et. al.The Ras/Raf/MEK/ERK signaling pathway (Figure 1) and its role in the occurrence and development of HCC. Oncology letters, 12(5), 3045–3050. DOI:10.3892/ol.2016.5110.
- ↑ 4.0 4.1 4.2 4.3 Hauseman, Z.J., Fodor, M., Dhembi, A. et al. Structure of the MRAS–SHOC2–PP1C phosphatase complex. Nature 609, 416–423 (2022). doi: 10.1038/s41586-022-05086-1. DOI:10.1038/s41586-022-05086-1.
- ↑ 5.0 5.1 Kwon, J. J., & Hahn, W. C. A Leucine-Rich Repeat Protein Provides a SHOC2 the RAS Circuit: a Structure-Function Perspective. Molecular and cellular biology, 41(4), e00627-20 (2021). doi:10.1128/MCB.00627-20. DOI: 10.1128/MCB.00627-20.
- ↑ Aggen, J., Nairn, A., Chamberlin, R. Regulation of protein phosphatase-1. Chemistry & Biology 2000, 7:R13–R23. [1].
- ↑ Zhou, Y., Prakash, P., Liang, H., et al. Lipid-Sorting Specificity Encoded in K-Ras Membrane Anchor Regulates Signal Output. Cell, 168(1-2), 239–251.e16 doi: 10.1016/j.cell.2016.11.059. DOI: 10.1016/j.cell.2016.11.059.
- ↑ Young, L., Rodriguez-Viciana, P. MRAS: A Close but Understudied Member of the RAS Family. Cold Spring Harbor Perspectives in Medicine (2018). doi: 10.1101/cshperspect.a033621. DOI: 0.1101/cshperspect.a033621.
- ↑ Daniel A. Bonsor, Patrick Alexander, Kelly Snead, Nicole Hartig, Matthew Drew, Simon Messing, Lorenzo I. Finci, Dwight V. Nissley, Frank McCormick, Dominic Esposito, Pablo Rodrigiguez-Viciana, Andrew G. Stephen, Dhirendra K. Simanshu. Structure of the SHOC2–MRAS–PP1C complex provides insights into RAF activation and Noonan syndrome. bioRxiv. 2022.05.10.491335. doi: 10.1101/2022.05.10.491335. DOI:10.1101/2022.05.10.491335.
- ↑ 10.0 10.1 10.2 Kwon, J., Jajian, B., Bian, Y. et al. Comprehensive structure-function evaluation of the SHOC2 holophosphatase reveals disease mechanisms and therapeutic opportunities. In: Proceedings of the American Association for Cancer Research Annual Meeting 2022. DOI: 10.1158/1538-7445.AM2022-LB029.
- ↑ 11.0 11.1 11.2 11.3 11.4 Kwon, J.J., Hajian, B., Bian, Y. et al. Structure–function analysis of the SHOC2–MRAS–PP1C holophosphatase complex. Nature 609, 408–415 (2022).doi: 10.1038/s41586-022-04928-2. DOI:10.1038/s41586-022-04928-2
- ↑ 12.0 12.1 Liau NPD, Johnson MC, Izadi S, Gerosa L, Hammel M, Bruning JM, Wendorff TJ, Phung W, Hymowitz SG, Sudhamsu J. Structural basis for SHOC2 modulation of RAS signalling. Nature. 2022 Jun 29. pii: 10.1038/s41586-022-04838-3. doi:, 10.1038/s41586-022-04838-3. PMID:35768504 doi:http://dx.doi.org/10.1038/s41586-022-04838-3
- ↑ 13.0 13.1 Lavoie, H., Therrien, M. Structural keys unlock RAS–MAPK cellular signaling pathway. Nature 609, 248-249 (2022). doi: 10.1038/d41586-022-02189-7. DOI:10.1038/d41586-022-02189-7.
- ↑ 14.0 14.1 van der Burgt, I. Noonan syndrome. Orphanet J Rare Dis 2, 4 (2007). doi: 10.1186/1750-1172-2-4 DOI: 10.1186/1750-1172-2-4.