Sandbox Reserved 1793
From Proteopedia
(Difference between revisions)
| (6 intermediate revisions not shown.) | |||
| Line 23: | Line 23: | ||
{| | {| | ||
|- | |- | ||
| - | | | + | | <html5media height="300" width="300">/Surface NTCP Movie.mp4 </html5media> |
| - | | [[ | + | | [[image:Surface_NTCP_morph.gif]] |
|- | |- | ||
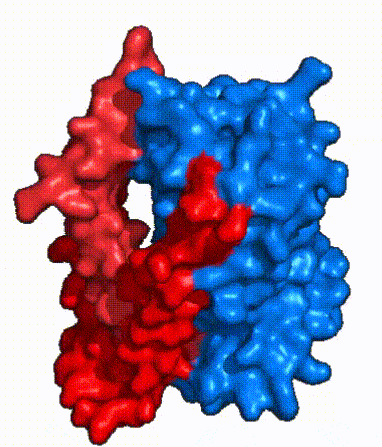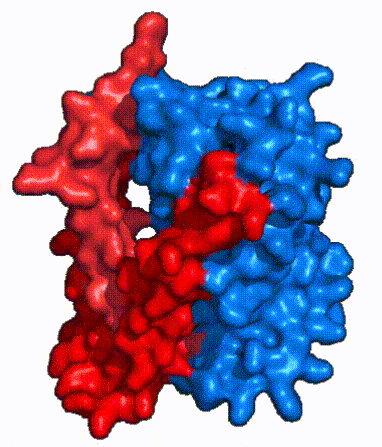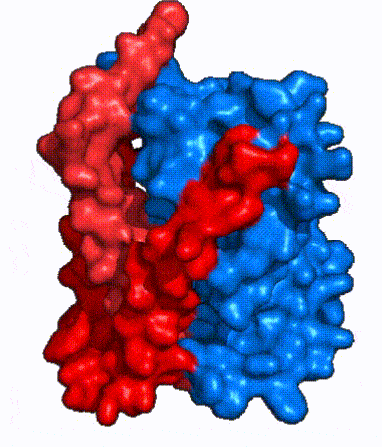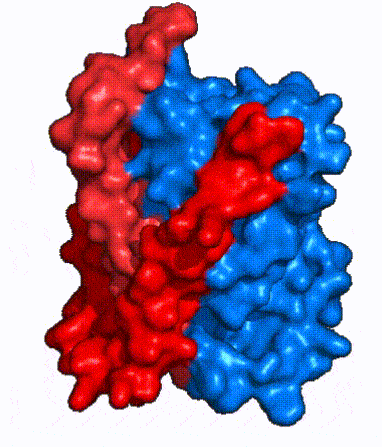
| Fig. 3 NTCP shown as cartoons with panel (red) and core (blue) domains colored. Helices are moving from open-pore to inward-facing conformation (7PQQ to 7PQG) | | Fig. 3 NTCP shown as cartoons with panel (red) and core (blue) domains colored. Helices are moving from open-pore to inward-facing conformation (7PQQ to 7PQG) | ||
| Line 35: | Line 35: | ||
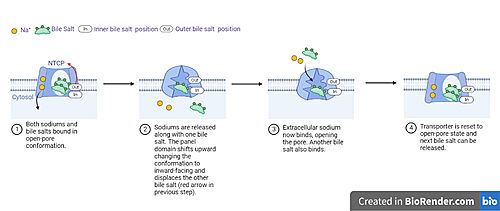
A proposed pathway for NTCP bile salt transport starting and ending with open-pore states hypothesizes that both sodium ions are translocated with the transport of one bile salt.<Ref name = "Liu"> Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). https://doi.org/10.1038/s41422-022-00680-4 </Ref>. Only one taurocholate is transported at a time due to NTCP's two bile salt binding sites. An | A proposed pathway for NTCP bile salt transport starting and ending with open-pore states hypothesizes that both sodium ions are translocated with the transport of one bile salt.<Ref name = "Liu"> Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). https://doi.org/10.1038/s41422-022-00680-4 </Ref>. Only one taurocholate is transported at a time due to NTCP's two bile salt binding sites. An | ||
| - | <scene name='95/952721/Inner_bile_salt/2'>inner bile salt</scene> that is closer to the cytoplasmic side of the membrane and an <scene name='95/952721/Outer_bile_salt/2'>outer bile salt</scene> that is closer to the extracellular side of the membrane <Ref name = "Liu"/> In the open-pore state both <scene name='95/952721/Mech_step_1/1'>taurocholates and sodium ions bound</scene> | + | <scene name='95/952721/Inner_bile_salt/2'>inner bile salt</scene> that is closer to the cytoplasmic side of the membrane and an <scene name='95/952721/Outer_bile_salt/2'>outer bile salt</scene> that is closer to the extracellular side of the membrane <Ref name = "Liu"/> In the open-pore state both <scene name='95/952721/Mech_step_1/1'>taurocholates and sodium ions bound.</scene> Next both sodium ions are released into the cytoplasm along with the inner bile salt into the cytoplasm (Fig. 5). Once the sodium ions are released this will trigger the conformational change in NTCP. The <scene name='95/952721/Mech_step_2/2'>outermost bile salt remains bound</scene> in the pore, likely helping to prevent leakage. <Ref name = "Liu"/> Then the <scene name='95/952721/Mech_step_3/2'> outer bile salt is displaced </scene> into the inner bile salt binding site by the movement from conformational change to the inward-facing, pore inaccessible state due to sodium ion release (Fig. 5). <Ref name = "Liu"/> Two new sodium ions then bind to NTCP from the extracellular side, favoring the open-pore state and also allowing for the binding of another outer bile salt (Fig 5). The <scene name='95/952721/Mech_step_1/1'>protein is then reset</scene> and the process can then start again releasing the next inner bile salt with the translocation of the sodium ions into the cytoplasm. This mechanism melds typical patterns of [https://proteopedia.org/wiki/index.php/Pump gated channels and pumps] in a new light as sodium ions control conformation and thus binding, displacement, and release of bile salts. <Ref name = "Goutam"/> |
== HBV Binding and Infection== | == HBV Binding and Infection== | ||
| - | NTCP is the only [https://rupress.org/jcb/article/195/7/1071/54877/The-cell-biology-of-receptor-mediated-virus entry receptor] <Ref name = "Grove"> Grove, J.; Marsh, M. The Cell Biology of Receptor-Mediated Virus Entry. Journal of Cell Biology 2011, 195 (7), 1071–1082. </ref> into the liver for HBV and HDV. <Ref name = "Asami"/>These viruses are known to use <scene name='95/952721/Hep_patches/2'>two different patches</scene> <font color='#00e080'><b>(residues 84-87 and 157-165)</b></font> on NTCP for binding and entry. The [https://en.wikipedia.org/wiki/Myristoylation myristolated] PreS1 domain of HBV binds to NTCP through the <scene name='95/952721/Hbv_patch_1/1'>first hydrophobic patch</scene> on NTCP containing <font color='#00e080'><b>residues 157-165</b></font> on the open pore surface. <Ref name = "Asami"/> These residues form part of the bile salt transport tunnel resulting in HBV binding and bile salt transport directly competing and interfering with one another. <Ref name = "Asami"/> The <scene name='95/952721/Hbv_patch_2/1'>other hydrophobic patch</scene> consisting of <font color='#00e080'><b>residues 84-87</b></font> found on the N-terminus of NTCP does not overlap with bile salt binding and may be used for the development of [https://en.wikipedia.org/wiki/Antiviral_drug antivirals] that do not inhibit bile uptake <Ref name = "Park"/>. Other minor variations within NTCP provide species specificity for HBV or virus resistance, such as mutant S267F found in East Asia. <Ref name = "Park"/> [ | + | NTCP is the only [https://rupress.org/jcb/article/195/7/1071/54877/The-cell-biology-of-receptor-mediated-virus entry receptor] <Ref name = "Grove"> Grove, J.; Marsh, M. The Cell Biology of Receptor-Mediated Virus Entry. Journal of Cell Biology 2011, 195 (7), 1071–1082. </ref> into the liver for HBV and HDV. <Ref name = "Asami"/>These viruses are known to use <scene name='95/952721/Hep_patches/2'>two different patches</scene> <font color='#00e080'><b>(residues 84-87 and 157-165)</b></font> on NTCP for binding and entry. The [https://en.wikipedia.org/wiki/Myristoylation myristolated] PreS1 domain of HBV binds to NTCP through the <scene name='95/952721/Hbv_patch_1/1'>first hydrophobic patch</scene> on NTCP containing <font color='#00e080'><b>residues 157-165</b></font> on the open pore surface. <Ref name = "Asami"/> These residues form part of the bile salt transport tunnel resulting in HBV binding and bile salt transport directly competing and interfering with one another. <Ref name = "Asami"/> The <scene name='95/952721/Hbv_patch_2/1'>other hydrophobic patch</scene> consisting of <font color='#00e080'><b>residues 84-87</b></font> found on the N-terminus of NTCP does not overlap with bile salt binding and may be used for the development of [https://en.wikipedia.org/wiki/Antiviral_drug antivirals] that do not inhibit bile uptake <Ref name = "Park"/>. Other minor variations within NTCP provide species specificity for HBV or virus resistance, such as mutant S267F found in East Asia. <Ref name = "Park"/> This S267F mutation is a [https://en.wikipedia.org/wiki/Single-nucleotide_polymorphism single-nucleotide polymorphism], where a change in one nucleotide in the sequence has caused a lack of bile salt transport activity or viral infection. <Ref name = "Park"/> It is hypothesized that due to the lack of bile salt transport in this mutation that some aspect or state of bile salt transport is necessary for HBV and HDV infection, suggesting the two functionally overlap. <Ref name = "Park"/> |
| - | The exact mechanism by which NTCP mediates viral internalization is still being determined; however, current evidence suggests it works through [https://en.wikipedia.org/wiki/Viral_entry#Entry_via_endocytosis endocytosis.] <Ref name = "Herrscher"> Herrscher C, Roingeard P, Blanchard E. Hepatitis B Virus Entry into Cells. Cells. 2020 Jun 18;9(6):1486. doi: 10.3390/cells9061486. PMID: 32570893; PMCID: PMC7349259. </ref> Once HBV is bound, the NTCP/HBV complex is taken into the cell where viral contents are dumped into the cytoplasm to then begin [https://en.wikipedia.org/wiki/Viral_replication viral replication]. HBV may also interact with other receptors or host cell factors, | + | The exact mechanism by which NTCP mediates viral internalization is still being determined; however, current evidence suggests it works through [https://en.wikipedia.org/wiki/Viral_entry#Entry_via_endocytosis endocytosis.] <Ref name = "Herrscher"> Herrscher C, Roingeard P, Blanchard E. Hepatitis B Virus Entry into Cells. Cells. 2020 Jun 18;9(6):1486. doi: 10.3390/cells9061486. PMID: 32570893; PMCID: PMC7349259. </ref> Once HBV is bound, the NTCP/HBV complex is taken into the cell where viral contents are dumped into the cytoplasm to then begin [https://en.wikipedia.org/wiki/Viral_replication viral replication]. HBV may also interact with other receptors or host cell factors, as cells overexpressing NTCP alone had low infection efficiency. <Ref name = "Herrscher"/> |
Current revision
Sodium Bile Salt Co-Transporting Protein
| |||||||||||
References
- ↑ Stieger B. The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb Exp Pharmacol. 2011;(201):205-59. doi: 10.1007/978-3-642-14541-4_5. PMID: 21103971. DOI: DOI: 10.1007/978-3-642-14541-4_5.
- ↑ Geyer, J., Wilke, T. & Petzinger, E. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmied Arch Pharmacol 372, 413–431 (2006). https://doi.org/10.1007/s00210-006-0043-8
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). DOI: 10.1038/s41586-022-04723-z.
- ↑ 4.0 4.1 4.2 4.3 4.4 Park, JH., Iwamoto, M., Yun, JH. et al. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature 606, 1027–1031 (2022). https://doi.org/10.1038/s41586-022-04857-0.
- ↑ Qi X. and Li W. (2022). Unlocking the secrets to human NTCP structure. The Innovation 3(5), 100294. https://doi.org/10.1016/j.xinn.2022.100294
- ↑ Latorraca, N. R.; Fastman, N. M.; Venkatakrishnan, A. J.; Frommer, W. B.; Dror, R. O.; Feng, L. Mechanism of Substrate Translocation in an Alternating Access Transporter. Cell 2017, 169 (1), 96–107.
- ↑ 7.0 7.1 7.2 7.3 Asami, J., Kimura, K.T., Fujita-Fujiharu, Y. et al.Structure of the bile acid transporter and HBV receptor NTCP. Nature 606, 1021–1026 (2022). https://doi.org/10.1038/s41586-022-04845-4
- ↑ 8.0 8.1 8.2 8.3 Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). https://doi.org/10.1038/s41422-022-00680-4
- ↑ Grove, J.; Marsh, M. The Cell Biology of Receptor-Mediated Virus Entry. Journal of Cell Biology 2011, 195 (7), 1071–1082.
- ↑ 10.0 10.1 Herrscher C, Roingeard P, Blanchard E. Hepatitis B Virus Entry into Cells. Cells. 2020 Jun 18;9(6):1486. doi: 10.3390/cells9061486. PMID: 32570893; PMCID: PMC7349259.