Sandbox Reserved 1789
From Proteopedia
(Difference between revisions)
| (3 intermediate revisions not shown.) | |||
| Line 11: | Line 11: | ||
== Introduction == | == Introduction == | ||
| - | <scene name='95/ | + | <scene name='95/952717/Zoom_out/1'>SHOC2-PP1C-MRAS</scene> (SMP) is a ternary holophosphotase complex formed by the individual proteins: SHOC2, PP1C, and MRAS. The SMP complex is involved in signaling the initiation of [https://www.nature.com/articles/7290105. MAPK pathways], which is responsible for cellular growth and development, cell proliferation, and apoptosis <ref name="Hauseman">PMID:35830882</ref>. Formation of this complex begins with an extracellular signal binding to a membrane embedded receptor [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2536775/. tyrosine kinase receptor(RTK)] <ref name="Hauseman"/>. This causes membrane-bound MRAS to exchange GDP for GTP. Initiating the SMP complex formation at the plasma membrane consists of the SHOC2 and PP1C binding first. When the MRAS exchanges GDP to GTP, it then assembles with the combined SHOC2 and PP1C. Based on MRAS targeting, PP1C catalyzes the [https://www.cell.com/fulltext/S0092-8674(00)81356-2. dephosphorylation] of the N-terminal phosphoserine (NTpS) on the [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3128629/. RAF complex] leading to the amplification of MAPK signaling <ref name="Hauseman"/>. In a normal cell, this would regulate cell proliferation but dysfunction in the ternary complex has shown signs to lead to tumor formation due to unregulated cell growth <ref name="Hauseman"/>. |
[[Image:Mechanism.png|200px|center|thumb|Figure 1: Mechanism for Shoc2-MRAS-PP1C]] | [[Image:Mechanism.png|200px|center|thumb|Figure 1: Mechanism for Shoc2-MRAS-PP1C]] | ||
| Line 21: | Line 21: | ||
<scene name='95/952717/Shoc2/1'>SHOC2</scene> is a scaffold protein composed of 20 [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3901792/. leucine-rich repeat (LRR)] domains that form a solenoid structure <ref name="Hauseman"/>. The leucine rich region forms a concave hydrophobic core which is necessary for binding with PP1C and MRAS. SHOC2 is the crucial mediator for SHOC2-PP1C-MRAS complex formation <ref name="Hauseman"/>. The leucine rich domain is very important in creating selectivity for the PP1C protein, as that protein is used for so many other complex pathways <ref name="Hauseman"/>. The LRR domains are stabilized by an N-terminal flanking 𝝰-helix and a C-terminal helix-turn-helix <ref name="Kwon">PMID:35831509</ref>. Alongside the conserved leucine residues in the LRR domain, there is a group of conserved asparagine residues that creates a stabilizing [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7184636/. “asparagine ladder”] that is necessary for the LRR fold, giving the SHOC2 its concave structure <ref name="Kwon" />. | <scene name='95/952717/Shoc2/1'>SHOC2</scene> is a scaffold protein composed of 20 [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3901792/. leucine-rich repeat (LRR)] domains that form a solenoid structure <ref name="Hauseman"/>. The leucine rich region forms a concave hydrophobic core which is necessary for binding with PP1C and MRAS. SHOC2 is the crucial mediator for SHOC2-PP1C-MRAS complex formation <ref name="Hauseman"/>. The leucine rich domain is very important in creating selectivity for the PP1C protein, as that protein is used for so many other complex pathways <ref name="Hauseman"/>. The LRR domains are stabilized by an N-terminal flanking 𝝰-helix and a C-terminal helix-turn-helix <ref name="Kwon">PMID:35831509</ref>. Alongside the conserved leucine residues in the LRR domain, there is a group of conserved asparagine residues that creates a stabilizing [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7184636/. “asparagine ladder”] that is necessary for the LRR fold, giving the SHOC2 its concave structure <ref name="Kwon" />. | ||
| - | SHOC2 is also capable of causing various forms of | + | SHOC2 is also capable of causing various forms of cancers and rasopathies. A common one is caused by a mutation known as p.S2G <ref name="Rauen">Rauen KA. The RASopathies. Annu Rev Genomics Hum Genet. 2013;14:355-69. [http://dx.doi.org/10.1146/annurev-genom-091212-153523 doi: 10.1146/annurev-genom-091212-153523.]</ref>. This mutation causes the formation of an additional 14-carbon saturated fatty acid chain on the N-terminal glycine of SHOC2 <ref name="Rauen" />. This causes SHOC2 to become attached to the cell membrane, resulting in a prolonged dephosphorylation of RAF by PP1C <ref name="Rauen" />. With this abnormality, there is overexpression of the MAPK pathway and increased cell proliferation genes <ref name="Rauen" />. This can cause the formation of various tumors in the body. Other mutations of the SMP ternary structure as a whole can also lead to the development of Noonan syndrome <ref name="Hauseman"/>. |
=== PP1C === | === PP1C === | ||
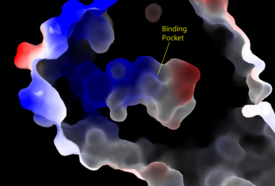
| - | <scene name='95/952717/Pp1c/ | + | <scene name='95/952717/Pp1c/2'>PP1C</scene> is a phosphatase. After forming a ternary complex, the hydrophobic active site on PP1C interacts with Raf and dephosphorylate Ser 259. PP1C's active site is adjacent to a hydrophobic binding pocket that binds to the C-terminal phosphoserine, located on the N-terminus of RAF, the target for dephosphorylation. PP1C can act as a phosphatase in the absence of SHOC2 but PP1C lacks intrinsic substrate selectively. The SMP complex formation endows PP1C with specificity for RAF <ref name="Hauseman" />. |
| + | |||
| + | The mechanism that PP1C uses to catalyze the dephosphorylation is mainly through donating a hydrogen atom to a phosphate group on the C-terminal of a phosphoserine on Raf <ref name="Kubicek"/>. This makes the phosphate group a good leaving group and it breaks off <ref name="Kubicek"/>. This catalysis is done by the serine-threonine alpha catalytic site on PP1C <ref name="Kubicek"/>. In this catalytic site, there are two Manganese ions and one calcium ion <ref name="Kubicek"/>. These metal ions are necessary in stablizing this catalytic site and there are a lot of polar negative residues in this region <ref name="Kubicek"/>. | ||
| + | |||
| + | The catalytic site is also capable of causing various rasopathies if there is a mutation present <ref name="Rauen" />. Typically the mutation is centered around the catalytic site not being able to attach to the dephosphorylation site on Ras <ref name="Rauen" />. This causes an underproduction of cell proliferation pathways and leads to Rasopathies. RASopathy is a broad term used to describe developmental syndromes that stem from germline mutations of proteins along the RAS/MAPK pathway. These mutations can be either gain or loss of function. Rasopathies can also lead to cancer <ref name="Rauen" />. The mutation in PP1C can result in damages in growth and development in multiple areas of the body .<ref name="Rauen" /> | ||
| - | The mechanism that PP1C uses to catalyze the dephosphorylation is mainly through donating a hydrogen atom to a phosphate group on the C-terminal of a [https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/phosphoserine. phosphoserine] on Raf <ref name="Kubicek"/>. This makes the phosphate group a good leaving group and it breaks off <ref name="Kubicek"/>. This catalysis is done by the serine-threonine alpha catalytic site on PP1C <ref name="Kubicek"/>. In this catalytic site, there are two Manganese ions and one calcium ion <ref name="Kubicek"/>. These metal ions are necessary in stablizing this catalytic site and there are a lot of polar negative residues in this region <ref name="Kubicek"/> | ||
| - | <scene name='95/952717/Pp1c/1'>PP1C</scene> | ||
=== MRAS === | === MRAS === | ||
Current revision
This page, as it appeared on June 14, 2016, was featured in this article in the journal Biochemistry and Molecular Biology Education.
SHOC2-PP1C-MRAS
| |||||||||||
Student Contributors
Madeline Gilbert Inaya Patel Rushda Hussein