User:Marcella Maringolo/Sandbox 1
From Proteopedia
| Line 9: | Line 9: | ||
[[Image: Diferences.jpg]] | [[Image: Diferences.jpg]] | ||
| + | |||
| + | Figure adapted from Nardone ''et al'', 2017. | ||
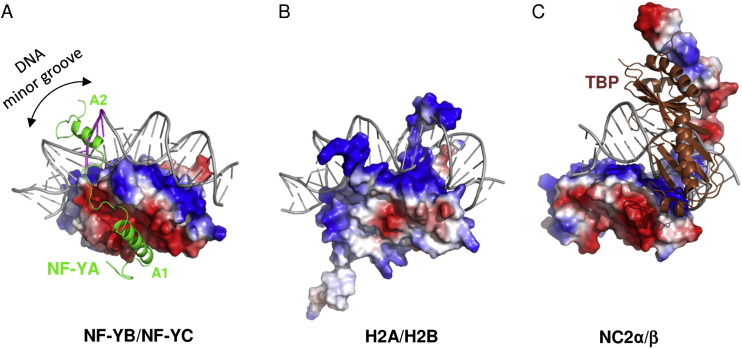
The heterodimerization of NF-YB and NF-YC allow NF-YA to associate with the complex, through binding of its A1 helix to NF-YB and NF-YC HFD domains, then allowing binding to CCAAT. NF-YA has a specific C-terminal domain responsible for CCAAT recognition, its A2 helix, which searches for the DNA motif for binding, but all of the 3 subunits can contact DNA after CCAAT binding. The contact regions for NF-Y subunits are shown in the image below. | The heterodimerization of NF-YB and NF-YC allow NF-YA to associate with the complex, through binding of its A1 helix to NF-YB and NF-YC HFD domains, then allowing binding to CCAAT. NF-YA has a specific C-terminal domain responsible for CCAAT recognition, its A2 helix, which searches for the DNA motif for binding, but all of the 3 subunits can contact DNA after CCAAT binding. The contact regions for NF-Y subunits are shown in the image below. | ||
[[Image:NF-Y DNA.jpg]] | [[Image:NF-Y DNA.jpg]] | ||
| + | |||
| + | Figure adapted from Nardini ''et al'', 2013. | ||
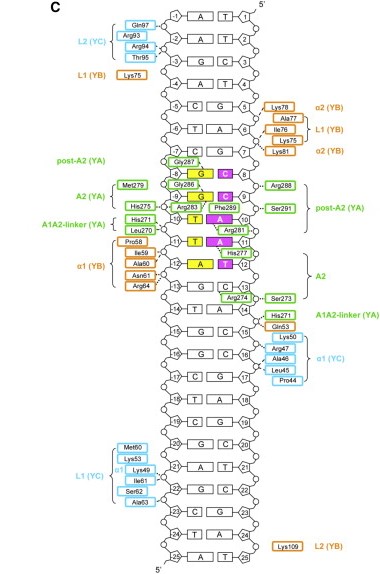
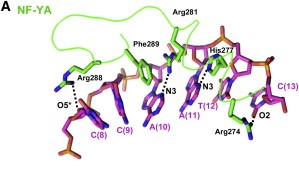
After finding the motif, NF-YA inserts its A2 helix into the DNA minor groove, which causes a bend in the DNA, allowing, then, other transcriptional factors to bind in adjacent DNA grooves. The binding of NF-YA is shown in the image below. | After finding the motif, NF-YA inserts its A2 helix into the DNA minor groove, which causes a bend in the DNA, allowing, then, other transcriptional factors to bind in adjacent DNA grooves. The binding of NF-YA is shown in the image below. | ||
[[Image:NF-YA.jpg]] | [[Image:NF-YA.jpg]] | ||
| + | |||
| + | Figure adapted from Nardini ''et al'', 2013. | ||
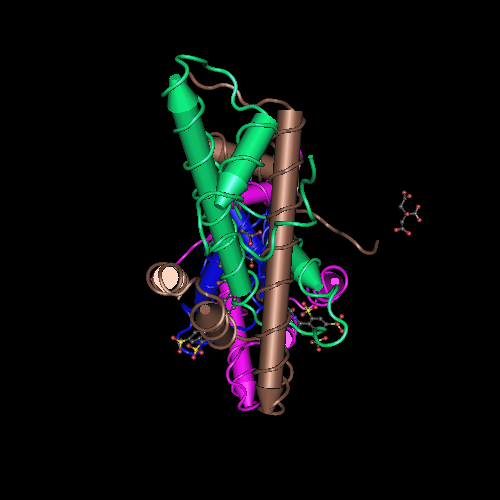
It was hypothesized that NF-YA is recruited by the HFD domain because it would function as a histone tail to allow and stabilize DNA binding with the NF-Y complex. Indeed, the core structures the histone dimer H2A/H2B overlay with NF-YB and NF-YC, while NF-YA overlay with the HB2 tail, indicating a similar function for those structures. | It was hypothesized that NF-YA is recruited by the HFD domain because it would function as a histone tail to allow and stabilize DNA binding with the NF-Y complex. Indeed, the core structures the histone dimer H2A/H2B overlay with NF-YB and NF-YC, while NF-YA overlay with the HB2 tail, indicating a similar function for those structures. | ||
| Line 36: | Line 42: | ||
[[Image: Mmdbimage.png]] | [[Image: Mmdbimage.png]] | ||
| + | Figure adapted from Nardone''et al'', 2020. | ||
</StructureSection> | </StructureSection> | ||
Current revision
| |||||||||||
References
Dolfini D, Gatta R, Mantovani R. NF-Y and the transcriptional activation of CCAAT promoters. Crit Rev Biochem Mol Biol. 2012 Jan-Feb;47(1):29-49. doi: 10.3109/10409238.2011.628970.
McNabb DS, Xing Y, Guarente L. Cloning of yeast HAP5: a novel subunit of a heterotrimeric complex required for CCAAT binding. Genes & Development. 1995 Jan;9(1):47-58. DOI: 10.1101/gad.9.1.47.
Nardini, Marco, et al. “Sequence-Specific Transcription Factor NF-Y Displays Histone-like DNA Binding and H2B-like Ubiquitination.” Cell, vol. 152, no. 1-2, 17 Jan. 2013, pp. 132–143, https://doi.org/10.1016/j.cell.2012.11.047.
Oldfield AJ, Henriques T, Kumar D, Burkholder AB, Cinghu S, Paulet D, Bennett BD, Yang P, Scruggs BS, Lavender CA, Rivals E, Adelman K, Jothi R. NF-Y controls fidelity of transcription initiation at gene promoters through maintenance of the nucleosome-depleted region. Nat Commun. 2019 Jul 11;10(1):3072. doi: 10.1038/s41467-019-10905-7.
Structural Basis of Inhibition of the Pioneer Transcription Factor NF-Y by Suramin Nardone V, Chaves-Sanjuan A, Lapi M, Airoldi C, Saponaro A, Pasqualato S, Dolfini D, Camilloni C, Bernardini A, Gnesutta N, Mantovani R, Nardini M