User:Daniel Key Takemoto/Sandbox 1
From Proteopedia
m |
|||
| (2 intermediate revisions not shown.) | |||
| Line 43: | Line 43: | ||
==Central portion== | ==Central portion== | ||
| - | The central portion of the protein contains the ''in tandem'' <scene name='96/969643/Kh1_kh2/1'>KH1 AND KH2 domains</scene>. The type of KH domains in eukaryots is type 1 fold | + | The central portion of the protein contains the ''in tandem'' <scene name='96/969643/Kh1_kh2/1'>KH1 AND KH2 domains</scene>. The type of KH domains in eukaryots is type 1 fold, in which a <scene name='96/969643/Sheets_helices_kh1_kh2/1'>β sheet composed of three antiparallel strands is locked by three helices</scene>, and they both have a canonical <scene name='96/969643/Gxxg/1'>G-X-X-G</scene> (glycine followed by any two aminoacids and another glycine) conserved domain, highlighted in magenta, connecting the central helices of the domain and a variable loop. There is a gap in the KH motif, which can hold '''four nucleic acid bases'''; it happens when an alpha-helix 1, alpha-helix 2, a GXXG on the left, and a variable loop are present in the domain, in the case of the FMRP, another beta-sheet. This helps the FMRP in its RBP function, as they are motifs that are related to '''RNA or ssDNA binding'''. <ref> Valverde, R., Edwards, L. and Regan, L. (2008). Structure and function of KH domains. FEBS Journal, 275(11), pp.2712–2726. [https://doi.org/10.1111/j.1742-4658.2008.06411.x]</ref> |
---- | ---- | ||
==RGG motif== | ==RGG motif== | ||
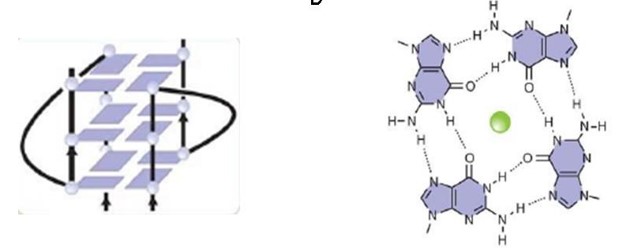
| - | An important motif of the FMRP is the <scene name='96/969643/Rgg_box/1'>RGG box</scene>, which the protein uses to bind to guanine G-quadruplexes, a structure that consists of nucleic acid folding in which four guanines arrange in a planar conformation stabilized by Hoogsteen- | + | An important motif of the FMRP is the <scene name='96/969643/Rgg_box/1'>RGG box</scene>, which the protein uses to bind to guanine G-quadruplexes, a structure that consists of nucleic acid folding in which four guanines arrange in a planar conformation stabilized by Hoogsteen-type hydrogen bonds, named tetrad, and they are stabilized by a K+ cation (green ball in the image). The RNA being represented is the ''sc1'' RNA, and the interaction between them was visualized through NMR. There are <scene name='96/969643/Sc1_gquadruplexes/1'>three G-Quadruplexes</scene> in the ''sc1'' RNA. FMRP RGG motifs seem to prefer binding to specific structures. |
Different domains and motifs mediate the RNA binding mechanism, and the exon 15-encoded <scene name='96/969643/Rgg_motif/2'>RGG motif</scene> (arginine - glycine - glycine) and the binding of FMRP to G-rich RNAs in vitro requires only the RGG motif, which occurs through the binding of a '''hydrogen bond between an RNA base and a aminoacid residue'''. The FMRP RGG motif is located in the C-terminal region of the protein and is well conserved in vertebrates. Crystal structure of the complex between the human FMRP RGG motif and G-quadruplex RNA The RGG motif binds to G-quadruplexes when it adopts a sharp turn and specifically binds to guanines from two consecutive G-C base pairs in the duplex-quadruplex junction. Several tetrads can stack in a single G-quadruplex structure and be stabilized further by potassium cations, in the case of FMRP targets, whereas they are destabilized by lithium cations. | Different domains and motifs mediate the RNA binding mechanism, and the exon 15-encoded <scene name='96/969643/Rgg_motif/2'>RGG motif</scene> (arginine - glycine - glycine) and the binding of FMRP to G-rich RNAs in vitro requires only the RGG motif, which occurs through the binding of a '''hydrogen bond between an RNA base and a aminoacid residue'''. The FMRP RGG motif is located in the C-terminal region of the protein and is well conserved in vertebrates. Crystal structure of the complex between the human FMRP RGG motif and G-quadruplex RNA The RGG motif binds to G-quadruplexes when it adopts a sharp turn and specifically binds to guanines from two consecutive G-C base pairs in the duplex-quadruplex junction. Several tetrads can stack in a single G-quadruplex structure and be stabilized further by potassium cations, in the case of FMRP targets, whereas they are destabilized by lithium cations. | ||
Current revision
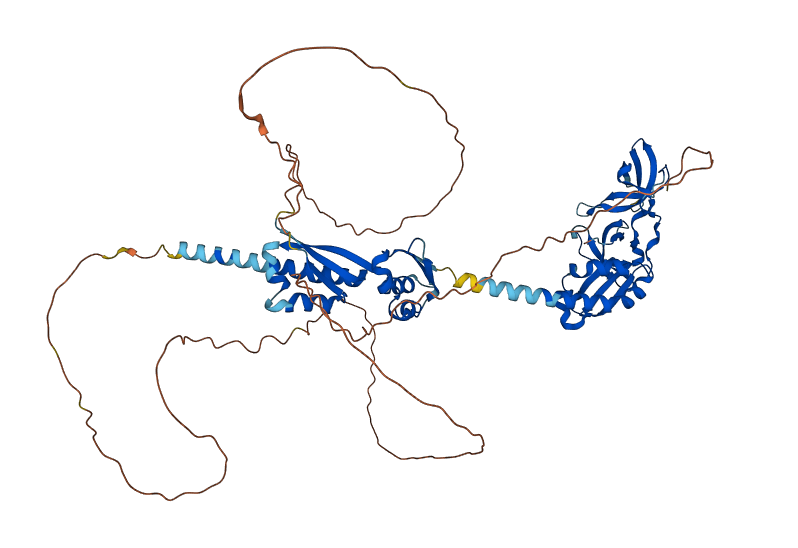
Structure of FMRP
Predicted FMRP
Fragile X messenger ribonucleoprotein (FMRP) is encoded by the fragile X messenger ribonucleoprotein 1 (FMR1) gene, located in the X chromosome, and is associated with the fragile X syndrome (FXS), Fragile X Tremor/Ataxia Syndrome (FXTAS), and Premature Ovarian Failure (POF1). FMRP functions as a synaptic regulator by binding to mRNAs and inhibiting its translation, therefore regulating the synthesis of proteins in the synapse. It is also an RNA binding protein, which is responsible for the transportation of mRNAs to the cytoplasm. The FMRP can also bind to its own FMR1 transcripts, possibly as a self-regulatory mechanism.
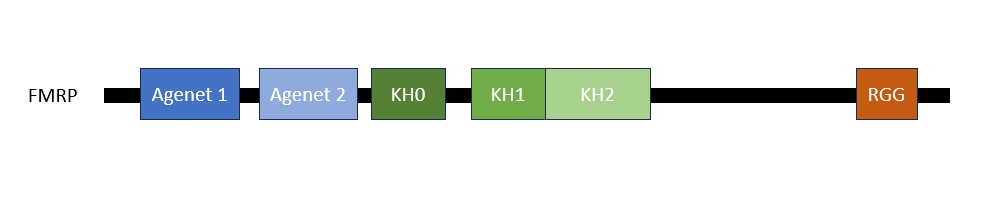
The FMRP is highly expressed in neurons and genitalia, and it's located mostly in the cytoplasm and at lower levels in the nucleus. It contains domains related to its RNA-binding function, either in the N-terminal or C-terminal regions; the Agenet and the KH0-domains are located in the N-terminal region, and they, respectively, exerce functions in binding to methylated lysin and RNA binding; the KH1 and KH2 motifs are located in the central region of the protein; and the RGG box, in the C-terminal region, acting as a binding to RNA, specifically to G-quadruplexes, a secondary RNA structure. The KH1, KH2, and RGG box domains allow the FMRP to bind and translate a number of mRNAs related to synaptic plasticity. [1]
The protein has 20 non-redundant isoforms and the most common is isoform 7, and the longest isoform contains 632 aminoacids. [2].
The predicted image was generated from Ensembl, by the AlphaFold program.
Overall structure
| |||||||||||