Nicotinic Acetylcholine Receptor
From Proteopedia
(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 29: | Line 29: | ||
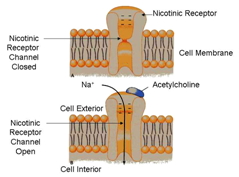
When in the presence of [[acetylcholine]], the receptor undergoes a conformational change opening up the channel to an influx of sodium (Na) within the cell. When this happens the cell undergoes a depolarization event that triggers an action potential to propagate along the rest of the cell stimulating, for example, a muscle response. | When in the presence of [[acetylcholine]], the receptor undergoes a conformational change opening up the channel to an influx of sodium (Na) within the cell. When this happens the cell undergoes a depolarization event that triggers an action potential to propagate along the rest of the cell stimulating, for example, a muscle response. | ||
| - | The opening of these channels only lasts for a millisecond due to [[cholinesterase]] being present and breaking down [[acetylcholine]] attached to the receptor causing the receptor to close again. Introduction of [[ | + | The opening of these channels only lasts for a millisecond due to [[cholinesterase]] being present and breaking down [[acetylcholine]] attached to the receptor causing the receptor to close again. Introduction of [[Acetylcholinesterase inhibitors]] can cause a depolarization block. It works by creating a prolonged refractory period in the depolarization event promoted by the opening of the receptor channel. |
=== Locations === | === Locations === | ||
| Line 79: | Line 79: | ||
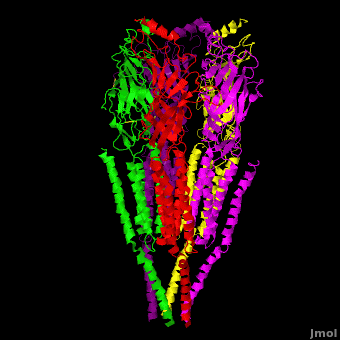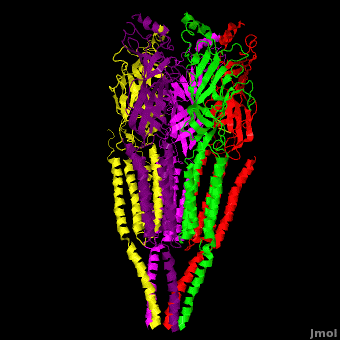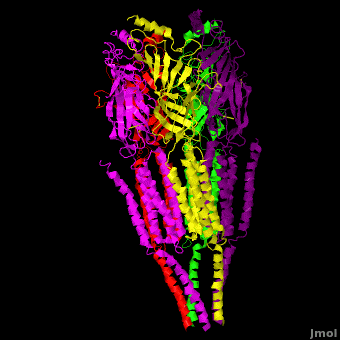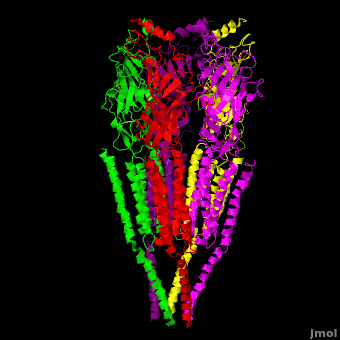
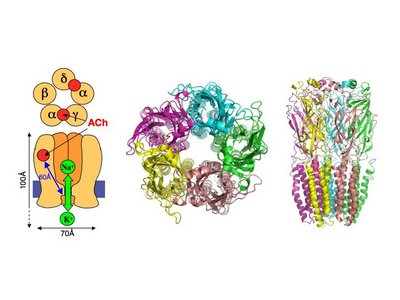
== 3D Structures of nicotinic acetylcholine receptor == | == 3D Structures of nicotinic acetylcholine receptor == | ||
| - | [[Acetyl choline receptor 3D structures | + | [[Acetyl choline receptor 3D structures]] |
</StructureSection> | </StructureSection> | ||
| - | == 3D Structures of nicotinic acetylcholine receptor == | ||
| - | |||
| - | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | |||
| - | [[4d01]] – hAChR α-9 subunit extracellular domain – human<br /> | ||
| - | [[4uxu]] – hAChR α-9 subunit extracellular domain + methyllycaconitine<br /> | ||
| - | [[4uy2]] – hAChR α-9 subunit extracellular domain + α-bungarotoxin<br /> | ||
| - | [[6hy7]] – hAChR alpha-9 subunit extracellular domain + α-conotoxin<br /> | ||
| - | [[5hbt]] – hAChR α-1 subunit extracellular domain + α-bungarotoxin + antibody<br /> | ||
| - | [[2qc1]] – AChR α subunit extracellular domain + α-bungarotoxin - mouse<br /> | ||
| - | [[3mra]] – TcAChR α subunit M3 transmembrane segment – ''Torpedo californica'' - NMR<br /> | ||
| - | [[2k58]] – AChR β-2 subunit M1 transmembrane segment - NMR<br /> | ||
| - | [[2k59]] – AChR β-2 subunit M2 transmembrane segment - NMR<br /> | ||
| - | [[1oed]] – TmAChR pore – ''Torpedo marmorata'' – electron images<br /> | ||
| - | [[2bg9]], [[4aq5]], [[4aq9]], [[4bog]], [[4boi]], [[4bon]], [[4boo]], [[4bor]], [[4bot]] – TmAChR – Cryo-EM<br /> | ||
== References == | == References == | ||
Current revision
| |||||||||||
References
1. Cholinesterase Inhibitors: Including Insecticides and Chemical Warfare Nerve Agents, Agency for Toxiz Substances and Disease Regulation accessed 5/2/14
2. Pierre-Jean Corringer and Jean-Pierre Changeux (2008) Nicotinic acetylcholine receptors. Scholarpedia, 3(1):3468.
3. Adcock C, Smith GR, Sansom MS. The nicotinic acetylcholine receptor: from molecular model to single-channel conductance. Eur Biophys J. 2000;29:29–37.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Joel L. Sussman, Alexander Berchansky, Alex Pennington