From Proteopedia
< User:Elizabeth Yowell(Difference between revisions)
proteopedia linkproteopedia link
|
|
| (10 intermediate revisions not shown.) |
| Line 8: |
Line 8: |
| | SARS-CoV-2 better known as Covid-19 sent the world into a global pandemic due to its rapid transmission and infection <ref name=”WHO”>https://www.who.int/europe/emergencies/situations/covid-19</ref>. In order to create a vaccine, it was essential to understand how SARS-CoV-2 infected us. | | SARS-CoV-2 better known as Covid-19 sent the world into a global pandemic due to its rapid transmission and infection <ref name=”WHO”>https://www.who.int/europe/emergencies/situations/covid-19</ref>. In order to create a vaccine, it was essential to understand how SARS-CoV-2 infected us. |
| | | | |
| - | | + | The enzyme angiotensin converting enzyme 2 <scene name='10/1077473/Ace2_only/1'>(ACE2)</scene> is attached to our cell membranes and then can be bound to a receptor binding domain <scene name='10/1077473/Rbd_only/2'>(RBD)</scene> <ref name="Borkotoky">DOI:10.1007/s11033-022-08193-4</ref>. The spike protein of SARS-CoV-2 enters our bodies and <scene name='10/1076049/Ace2andrbd/1'>binds to the RBD</scene>, allowing the spike protein access to ACE2 and our host cells <ref name=”Jackson”>DOI:10.1038/s41580-021-00418-x</ref>. Once the spike protein has access to our host cells, it is able to further infect our cells and spread the virus throughout our bodies, causing us to get sick. In order to create an effective vaccine, the pathway between the spike protein and the RBD needed to be [https://www.sciencedirect.com/science/article/pii/S0166354223000190 interrupted]. |
| - | The enzyme angiotensin converting enzyme 2 <scene name='10/1076049/Ace2andrbd/1'>(ACE2)</scene> is attached to our cell membranes and then can be bound to a receptor binding domain (RBD) <ref name="Borkotoky">DOI:10.1007/s11033-022-08193-4</ref>. The spike protein of SARS-CoV-2 enters our bodies and binds to the RBD, allowing the spike protein access to ACE2 and our host cells <ref name=”Jackson”>DOI:10.1038/s41580-021-00418-x</ref>. Once the spike protein has access to our host cells, it is able to further infect our cells and spread the virus throughout our bodies, causing us to get sick. In order to create an effective vaccine, the pathway between the spike protein and the RBD needed to be [https://www.sciencedirect.com/science/article/pii/S0166354223000190 interrupted]. | + | |
| | | | |
| | | | |
| Line 20: |
Line 19: |
| | | | |
| | ==Vaccines== | | ==Vaccines== |
| - | A common way to create vaccines is through the usage of antibodies.<scene name='10/1077473/Antibody_overview/2'>Antibodies</scene> are inserted into the body that mimic ACE2, due to the similarities with ACE2, when COVID-19 spike protein enters the body, they <scene name='10/1077473/Antibody/2'>bind</scene> to these ACE2 mimicking antibodies that create a 90% neutralizing response for targeting the RBD. With this being said, this method of treatment is difficult for long term use due to the evolution of the viral cells <ref name="Zhang">DOI:10.1016/S2666-5247(23)00011-3</ref>. | + | A common way to create vaccines is through the usage of antibodies.<scene name='10/1077473/Antibody_overview/2'>Antibodies</scene> are inserted into the body that mimic ACE2, due to the similarities with ACE2, when COVID-19 spike protein enters the body, they <scene name='10/1077473/Antibody/4'>bind</scene> to these ACE2 mimicking antibodies that create a 90% neutralizing response for targeting the RBD. With this being said, this method of treatment is difficult for long term use due to the evolution of the viral cells <ref name="Zhang">DOI:10.1016/S2666-5247(23)00011-3</ref>. |
| | | | |
| - | ===AHB2 and LCB1/LCB3 Specifically=== | + | ==Inhibitor Development== |
| | Protein inhibitors were thought of as a new idea for creating vaccines due to their smaller size and better stability compared to antibody vaccines<ref name="Cao">DOI:10.1126/science.abd9909</ref>. These protein inhibitors are also referred to as mini-binders, they interact with the ACE2 receptor binding domain, <scene name='10/1075220/Spikeblockedbyminibinder/2'>preventing association of the viral cell with ACE-2.</scene> | | Protein inhibitors were thought of as a new idea for creating vaccines due to their smaller size and better stability compared to antibody vaccines<ref name="Cao">DOI:10.1126/science.abd9909</ref>. These protein inhibitors are also referred to as mini-binders, they interact with the ACE2 receptor binding domain, <scene name='10/1075220/Spikeblockedbyminibinder/2'>preventing association of the viral cell with ACE-2.</scene> |
| | | | |
| Line 50: |
Line 49: |
| | ==Binding Site and Interactions== | | ==Binding Site and Interactions== |
| | | | |
| - | In order to best target the RBD of the spike protein, the minibinders reveal a wide range of interactions to compete with <scene name='10/1075219/Ace2/1'>ACE2 binding</scene>. ACE2's main interactions with the spike protein include D30, H34, Y41, D38, and E35 of ACE2 with K417, Y453, Q498, K353, and Q493 of the spike protein, respectively.
| + | <scene name='10/1075219/Lcb1/1'>LCB1 binding site</scene> |
| | | | |
| - | The first design method, Rosetta blueprint, created AHB2 based on the single interacting helix of ACE2. With <scene name='10/1075219/Ahb2/2'>AHB2 binding</scene>, we see two alpha helices mimicking ACE2. Hydrogen bonding interactions between N36, D11, K43, E41, and E30 of the minibinder interact with residues K417, R403, Y449, Q493, and N487, respectively, in the spike protein. One interesting result of the Rosetta blueprint design was the addition of the second helix to interact with the spike protein. It is assumed that this addition further stabilizes the interaction, increasing stability of the minibinder:spike complex.
| + | <scene name='10/1075219/Lcb3/2'>LCB3 binding site</scene> |
| | | | |
| - | De novo designed proteins, as discussed previously, focused on computational design to determine residues best able to interact with the spike protein. We will focus on LCB1 and LCB3. <scene name='10/1075219/Lcb1/4'>LCB1 binding</scene> reveals hydrogen bonding between D30 of the minibinder and both K417 and R403 of the spike protein, in addition to D17 and R14 of the minibinder interacting with Q493 of the spike protein. Similarly, <scene name='10/1075219/Lcb3/5'>LCB3 binding</scene> reveals hydrogen bonding between D11 of the minibinder to K417 and R403 of the spike protein.
| + | <scene name='10/1075219/Ahb2/1'>AHB2 binding site</scene> |
| | | | |
| - | Within all four binding sites, we see two spike protein residues interacting with all four minibinders: K417 and Q493. This suggests that these resides are critical for binding.
| + | <scene name='10/1075219/Ace2/1'>ACE2 binding site</scene> |
| | | | |
| | ==Inhibitor Differences== | | ==Inhibitor Differences== |
Current revision
Engineered Protein Inhibitors for SARS-CoV-2 Entry
|
Introduction
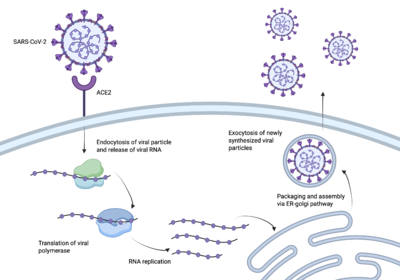 The process of the SARS-CoV-2 spike protein entering human cells. SARS-CoV-2 better known as Covid-19 sent the world into a global pandemic due to its rapid transmission and infection [1]. In order to create a vaccine, it was essential to understand how SARS-CoV-2 infected us.
The enzyme angiotensin converting enzyme 2 is attached to our cell membranes and then can be bound to a receptor binding domain [2]. The spike protein of SARS-CoV-2 enters our bodies and , allowing the spike protein access to ACE2 and our host cells [3]. Once the spike protein has access to our host cells, it is able to further infect our cells and spread the virus throughout our bodies, causing us to get sick. In order to create an effective vaccine, the pathway between the spike protein and the RBD needed to be interrupted.
Vaccines
A common way to create vaccines is through the usage of antibodies. are inserted into the body that mimic ACE2, due to the similarities with ACE2, when COVID-19 spike protein enters the body, they to these ACE2 mimicking antibodies that create a 90% neutralizing response for targeting the RBD. With this being said, this method of treatment is difficult for long term use due to the evolution of the viral cells [4].
Inhibitor Development
Protein inhibitors were thought of as a new idea for creating vaccines due to their smaller size and better stability compared to antibody vaccines[5]. These protein inhibitors are also referred to as mini-binders, they interact with the ACE2 receptor binding domain,
Their Discovery
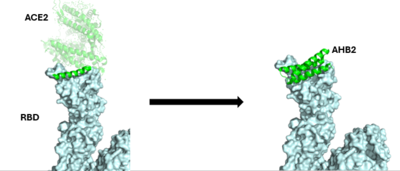 The use of the Rosetta Blueprint protein design to create the AHB2 inhibitor. The first mini-binder to be created to combat COVID-19 is called AHB2. In order to ensure that the mini-binder would bind to the same RBD that the ACE2 was bound to, AHB2 was designed by looking at sequence of ACE2 alpha-helix that makes interactions with spike receptor binding domain. This design process is referred to as the Rosetta Blueprint protein design [5].
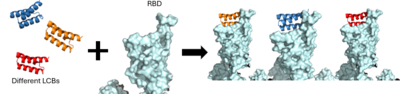 The use of the De Novo protein design to create the LCB1 and LCB3 inhibitors. As the AHB2 inhibitors were tested and found to be effective, it was then time to manipulate the mini-binders to create a more effective vaccine. A rotamer interaction field docking with in silico mini-proteins were used by using a scaffold library to generate binders to more distinct regions of the RBD surface [5]. This method is known as the de novo protein design and it is how the LCB1 and LCB3 mini-binders were created.
Binding Site and Interactions
Inhibitor Differences
 The sequence differences between ACE2, AHB2, LCB1and LCB3.
Stability
One of the most important findings with these DeNovo proteins is their high stability, allowing for less delicate forms of administration.
,
, and
|
References
Cao, L., Goreshnik, I., Coventry, B., Case, J.B., Miller, L., Kozodoy, L., Chen, R.E., Carter, L., Walls, A.C., Park, Y., Strauch, E., Stewart, L., Diamond, M.S., Veesler, D., & Baker, D. De novo design of picomolar SARS-CoV-2 mini protein inhibitors. Science 370, 426-431 (2020). https://doi.org/10.1126/science.abd9909
https://www.who.int/europe/emergencies/situations/covid-19
https://pmc.ncbi.nlm.nih.gov/articles/PMC9786537/#:~:text=The%20receptor%2Dbinding%20domain%20(RBD,that%20initiates%20the%20viral%20transmission.
https://www.nature.com/articles/s41580-021-00418-x#citeas
https://www.science.org/doi/10.1126/science.abd9909
Zhang, Haoran et al. Advances in developing ACE2 derivatives against SARS-CoV-2. The Lancet Microbe, Volume 4, Issue 5, e369 - e378 (2023). https://doi.org/10.1016/S2666-5247(23)00011-3
PDB Files
[1]https://www.rcsb.org/structure/7UHB
Student Contributors
- Giavanna Yowell
- Shea Bailey
- Matthew Pereira




