Sandbox Reserved 1845
From Proteopedia
(Difference between revisions)
| (16 intermediate revisions not shown.) | |||
| Line 23: | Line 23: | ||
=== Catalytic Triad === | === Catalytic Triad === | ||
[[Image:Final rayed image of binding pocket.png|400 px|right|thumb|Figure 1: Ser, His, Asp catalytic triad non-covalent stabilizing interactions with oxyanion hole.]] | [[Image:Final rayed image of binding pocket.png|400 px|right|thumb|Figure 1: Ser, His, Asp catalytic triad non-covalent stabilizing interactions with oxyanion hole.]] | ||
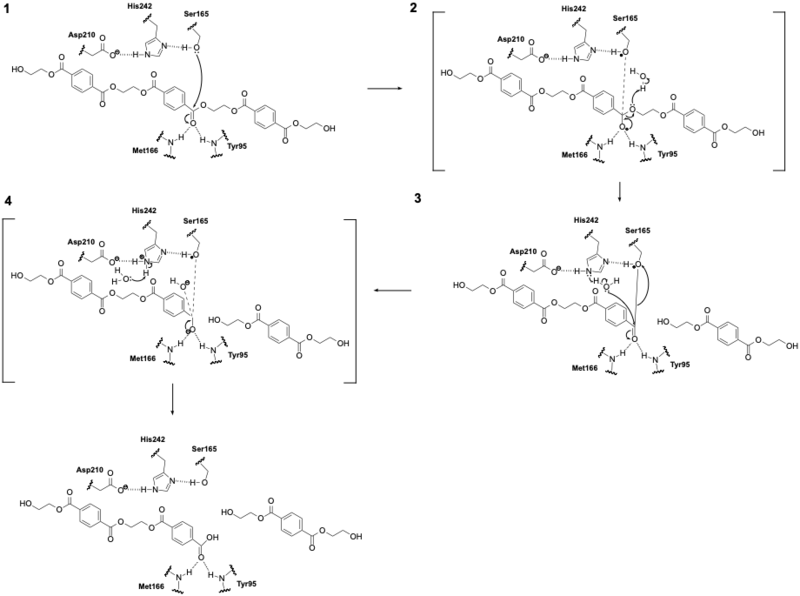
| - | LCC catalyzes the breakdown of PET using a serine hydrolase mechanism with a <scene name='10/1075247/Catalytic_triad3_w_label/3'>catalytic triad</scene> of Ser165, | + | LCC catalyzes the breakdown of PET using a serine hydrolase mechanism with a <scene name='10/1075247/Catalytic_triad3_w_label/3'>catalytic triad</scene> of Ser165, Asp210, and His242. (Figure 1) (1) The reaction begins when His242 deprotonates Ser165, which activates it as a nucleophile. (2) Ser165 then attacks the carbonyl carbon of an ester bond in the PET polymer to form a tetrahedral intermediate. (3) This tetrahedral intermediate is stabilized by an oxyanion hole formed by the backbone amides of Met166 and Tyr95. (4) The intermediate collapses; one product is released and an acyl-enzyme intermediate is formed. (5) A water molecule, activated by His242, then attacks the acyl-enzyme. This releases the second product and resets the enzyme’s active site. |
[[Image:Mech2.png|800 px|right|thumb|Figure 2: LCC mechanism. LCC hydrolyzes PET using a catalytic triad (Ser165, His242, Asp210) to cleave ester bonds via a tetrahedral intermediate.]] | [[Image:Mech2.png|800 px|right|thumb|Figure 2: LCC mechanism. LCC hydrolyzes PET using a catalytic triad (Ser165, His242, Asp210) to cleave ester bonds via a tetrahedral intermediate.]] | ||
| Line 32: | Line 32: | ||
== Mutation Sites of Interest == | == Mutation Sites of Interest == | ||
| - | To improve the catalytic activity and thermostability of LCC, Tournier et al. (2020) used structure-guided enzyme engineering based on the crystal structure of LCC bound to a model PET substrate. Using [https://en.wikipedia.org/wiki/Docking_(molecular) molecular docking] and enzyme–substrate contact analysis, the researchers identified 15 | + | To improve the catalytic activity and thermostability of LCC, Tournier et al. (2020) used structure-guided enzyme engineering based on the crystal structure of LCC bound to a model PET substrate. Using [https://en.wikipedia.org/wiki/Docking_(molecular) molecular docking] and enzyme–substrate contact analysis, the researchers identified <scene name='10/1075247/Original_15_mutation_structure/6'>15 Residues</scene> in the first contact shell surrounding the substrate-binding groove. Of these, 11 positions were selected for [https://en.wikipedia.org/wiki/Saturation_mutagenesis#:~:text=Saturation%20mutagenesis%2C%20or%20site%20saturation,amino%20acids%20at%20the%20position. saturation mutagenesis] to determine how mutations could affect PET depolymerization. These sites were chosen for their interactions with the PET-like ligand or their proximity to the active site. Highly conserved residues essential for catalysis or structural stability were excluded. Using [https://consurf.tau.ac.il/consurf_index.php. ConSurf] demonstrates the residues that are conserved over all versions of LCC, to determine what other variations have changed, seen in Figure 3. |
| + | [[Image:Conserved amino acids.jpg|400 px|right|thumb|Figure 3: Image of protein structure, amino acids are colored depending on how often they are conserved in structure. Legend is included. ]] | ||
From this screen, two mutations at Phe243 (F243I and F243W) were shown to improve catalytic activity by optimizing substrate positioning within the groove. To increase thermostability, the authors targeted a region of the enzyme that is structurally analogous to known divalent [https://en.wikipedia.org/wiki/Metal-binding_protein metal binding] sites in other cutinases. Instead of using stabilizing ions, which could complicate industrial degradation processes, they engineered a [https://en.wikipedia.org/wiki/Disulfide disulfide bridge] by mutating Asp238 and Ser283 to Cys residues (D238C/S283C). Additional mutations were selected based on thermostability screening. Among these, Y127G improved the melting point without reducing activity. | From this screen, two mutations at Phe243 (F243I and F243W) were shown to improve catalytic activity by optimizing substrate positioning within the groove. To increase thermostability, the authors targeted a region of the enzyme that is structurally analogous to known divalent [https://en.wikipedia.org/wiki/Metal-binding_protein metal binding] sites in other cutinases. Instead of using stabilizing ions, which could complicate industrial degradation processes, they engineered a [https://en.wikipedia.org/wiki/Disulfide disulfide bridge] by mutating Asp238 and Ser283 to Cys residues (D238C/S283C). Additional mutations were selected based on thermostability screening. Among these, Y127G improved the melting point without reducing activity. | ||
| + | |||
| + | === Phe243 === | ||
| + | |||
| + | <scene name='10/1075247/F243_original/1'>Phe243</scene> is located 3.6 Å from the ligand, <scene name='10/1075247/Original_15_mutation_structure/6'>Reference Phe243</scene>. Two mutations at this position, <scene name='10/1075247/F243i/1'>F243I</scene> and F243W, increase the catalytic activity of the enzyme. The F243I mutation inserts the smaller isoleucine whose side chain allows the ligand to sit closer. This reduces the ligand distance to 3.0 Å, improving substrate binding. The F243W mutation inserts the bulkier, nitrogen-containing aromatic aide chain. Trp brings the ligand slightly closer at 3.2 Å and introduces potential for new interactions, such as hydrogen bonding or [https://en.wikipedia.org/wiki/Pi-stacking#:~:text=In%20chemistry%2C%20pi%20stacking%20(also,interaction%22)%20is%20electrostatically%20repulsive. π-stacking]. Both mutations result in improved catalytic performance. The F243I mutant shows a 27.5% increase in activity, while the F243W mutant shows a 17.5% increase, compared to the wild-type enzyme.<ref name="Tournier"/> | ||
| + | |||
| + | === Tyr 127 === | ||
| + | The mutation of Tyr to Gly at <scene name='10/1075247/Y127/4'>Tyr127</scene> also increases the thermostability,<scene name='10/1075247/Original_15_mutation_structure/6'>Reference Tyr127</scene>. The melting point of Y127G is increased to 87.0°C from the WT melting point of 84.7°C. Tyr has a bulky, rigid aromatic side chain that can cause structural strain, shown in <scene name='10/1075247/Y127_spacefill/1'>Y127 representation</scene>. Gly is the smallest amino acid and lacks a side chain, providing greater flexibility. The mutation <scene name='10/1075247/Y127g/2'>Y127G</scene> melting point is increased to 87.0°C. The mutation reduces [https://en.wikipedia.org/wiki/Steric_effects#:~:text=Steric%20hindrance%20is%20the%20slowing,as%20slowing%20unwanted%20side%2Dreactions. steric hindrance] and relieves strain in the protein structure, demonstrated in <scene name='10/1075247/Y127g_space_fill/1'>Y127G representation</scene>. By increasing flexibility, the Y127G mutation helps the protein maintain its folded structure under heat stress.<ref name="Tournier"/> | ||
| + | |||
| + | === Ser 283 & Asp 238 === | ||
| + | Ser 283 and Asp 238, are located outside of the binding pocket in the <scene name='10/1075247/Start_material_for_s283_and_d2/2'>Reference Ser283 and Asp238</scene>.Two wild-type residues, <scene name='10/1075247/S283-d238/5'>Ser283 and Asp238</scene>, were engineered to form a disulfide bond by replacing them with Cys. This decision was based on their spatial proximity in the 3D structure and their location in a region that resembles metal-binding sites in homologous PET-degrading enzymes. Unlike those metal-dependent sites, the LCC structure lacked coordinated ions. For that reason, the researchers engineered a covalent linkage instead to increase thermal stability without requiring additives like calcium. The wild-type protein has a melting point of 84.7°C, while the <scene name='10/1075248/C283-c238/2'>S283C and D238C mutant</scene> increased the melting point to 94.5°C, a 9.8°C improvement, which is higher than any other mutations. However, this increase in stability was accompanied by a 28% decrease in enzymatic activity compared to the wild-type. This trade-off between stability and activity shows the balance in enzyme engineering, as increasing structural integrity can sometimes restrict the flexibility needed for catalytic function. | ||
| + | |||
| + | == Group Mutations == | ||
These mutations were combined to create multi-mutant LCC variants with improved activity and thermostability. The two most successful variants were: | These mutations were combined to create multi-mutant LCC variants with improved activity and thermostability. The two most successful variants were: | ||
ICCG: F243I / D238C / S283C / Y127G | ICCG: F243I / D238C / S283C / Y127G | ||
| Line 42: | Line 55: | ||
Other stabilizing mutations, such as T96M, N246D, and N246M, were also tested but are not included in this page's protein model. These were excluded because they were not part of the top-performing mutant (ICCG), and therefore omitted for clarity.<ref name="Tournier"/> | Other stabilizing mutations, such as T96M, N246D, and N246M, were also tested but are not included in this page's protein model. These were excluded because they were not part of the top-performing mutant (ICCG), and therefore omitted for clarity.<ref name="Tournier"/> | ||
| - | |||
| - | === F243 === | ||
| - | The original residue at <scene name='10/1075247/Better_f234/3'>F243</scene> was mutated. This residue is located 3.6 Å from the ligand. Two mutations at this position, <scene name='10/1075247/Better_f243i/2'>F243I</scene> and F243W, increase the catalytic activity of the enzyme. The F243I mutation replaces Phe with Ile, a smaller side chain that allows the ligand to sit closer. This reduces the ligand distance to 3.0 Å. This tighter interaction improves substrate binding. The F243W mutation contains Trp, which has a bulkier, nitrogen-containing aromatic side chain. Trp brings the ligand slightly closer at 3.2 Å and introduces potential for new interactions, such as hydrogen bonding or [https://en.wikipedia.org/wiki/Pi-stacking#:~:text=In%20chemistry%2C%20pi%20stacking%20(also,interaction%22)%20is%20electrostatically%20repulsive. π-stacking]. Both mutations result in improved catalytic performance. The F243I mutant shows a 27.5% increase in activity, while the F243W mutant shows a 17.5% increase, compared to the wild-type enzyme.<ref name="Tournier"/> | ||
| - | |||
| - | === Y127 === | ||
| - | The mutation of Tyr to Gly at <scene name='10/1075247/Y127/4'>Y127</scene> also increases the protein's thermostability. The mutant melting point is increased to 87.0°C. Tyr has a bulky, rigid aromatic side chain that can cause structural strain. Gly is the smallest amino acid and lacks a side chain, so it provides greater flexibility to the protein. The <scene name='10/1075247/Y127g/2'>Y127G</scene> mutation reduces [https://en.wikipedia.org/wiki/Steric_effects#:~:text=Steric%20hindrance%20is%20the%20slowing,as%20slowing%20unwanted%20side%2Dreactions. steric hindrance] and relieves strain in the protein structure. By increasing flexibility, the Y127G mutation helps the protein maintain its folded structure under heat stress.<ref name="Tournier"/> | ||
| - | |||
| - | === S283 & D238 === | ||
| - | Two wild-type residues, <scene name='10/1075247/S283-d238/5'>S283 and D238</scene>, were engineered to form a disulfide bond by replacing them with Cys. This decision was based on their spatial proximity in the 3D structure and their location in a region that resembles metal-binding sites in homologous PET-degrading enzymes. Unlike those metal-dependent sites, the LCC structure lacked coordinated ions. For that reason, the researchers engineered a covalent linkage instead to increase thermal stability without requiring additives like calcium. The wild-type protein has a melting point of 84.7°C, while the <scene name='10/1075248/C283-c238/2'>S283C and D238C mutant</scene> increased the melting point to 94.5°C, a 9.8°C improvement, which is higher than any other mutations. However, this increase in stability was accompanied by a 28% decrease in enzymatic activity compared to the wild-type. This trade-off between stability and activity shows the balance in enzyme engineering, as increasing structural integrity can sometimes restrict the flexibility needed for catalytic function. | ||
</StructureSection> | </StructureSection> | ||
Current revision
| This Sandbox is Reserved from March 18 through September 1, 2025 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson and Mark Macbeth at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1828 through Sandbox Reserved 1846. |
To get started:
More help: Help:Editing |
Leaf Branch Compost Cutinase
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Tournier V, Topham CM, Gilles A, David B, Folgoas C, Moya-Leclair E, Kamionka E, Desrousseaux ML, Texier H, Gavalda S, Cot M, Guemard E, Dalibey M, Nomme J, Cioci G, Barbe S, Chateau M, Andre I, Duquesne S, Marty A. An engineered PET depolymerase to break down and recycle plastic bottles. Nature. 2020 Apr;580(7802):216-219. doi: 10.1038/s41586-020-2149-4. Epub 2020 Apr, 8. PMID:32269349 doi:http://dx.doi.org/10.1038/s41586-020-2149-4
- ↑ 2.0 2.1 2.2 2.3 2.4 Sui B, Wang T, Fang J, Hou Z, Shu T, Lu Z, Liu F, Zhu Y. Recent advances in the biodegradation of polyethylene terephthalate with cutinase-like enzymes. Front Microbiol. 2023 Oct 2;14:1265139. PMID:37849919 doi:10.3389/fmicb.2023.1265139
- ↑ Ueda H, Tabata J, Seshime Y, Masaki K, Sameshima-Yamashita Y, Kitamoto H. Cutinase-like biodegradable plastic-degrading enzymes from phylloplane yeasts have cutinase activity. Biosci Biotechnol Biochem. 2021 Jul 23;85(8):1890-1898. PMID:34160605 doi:10.1093/bbb/zbab113
- ↑ Kolattukudy PE. Biopolyester membranes of plants: cutin and suberin. Science. 1980 May 30;208(4447):990-1000. PMID:17779010 doi:10.1126/science.208.4447.990
- ↑ 5.0 5.1 5.2 5.3 5.4 Khairul Anuar NFS, Huyop F, Ur-Rehman G, Abdullah F, Normi YM, Sabullah MK, Abdul Wahab R. An Overview into Polyethylene Terephthalate (PET) Hydrolases and Efforts in Tailoring Enzymes for Improved Plastic Degradation. Int J Mol Sci. 2022 Oct 20;23(20):12644. PMID:36293501 doi:10.3390/ijms232012644
- ↑ 6.0 6.1 Burgin T, Pollard BC, Knott BC, Mayes HB, Crowley MF, McGeehan JE, Beckham GT, Woodcock HL. The reaction mechanism of the Ideonella sakaiensis PETase enzyme. Commun Chem. 2024 Mar 27;7(1):65. PMID:38538850 doi:10.1038/s42004-024-01154-x
- ↑ 7.0 7.1 7.2 Zhang J, Wang H, Luo Z, Yang Z, Zhang Z, Wang P, Li M, Zhang Y, Feng Y, Lu D, Zhu Y. Computational design of highly efficient thermostable MHET hydrolases and dual enzyme system for PET recycling. Commun Biol. 2023 Nov 9;6(1):1135. PMID:37945666 doi:10.1038/s42003-023-05523-5
- ↑ Yoshida S, Hiraga K, Takehana T, Taniguchi I, Yamaji H, Maeda Y, Toyohara K, Miyamoto K, Kimura Y, Oda K. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science. 2016 Mar 11;351(6278):1196-9. doi: 10.1126/science.aad6359. PMID:26965627 doi:http://dx.doi.org/10.1126/science.aad6359
- ↑ Landrigan PJ, Stegeman JJ, Fleming LE, Allemand D, Anderson DM, Backer LC, Brucker-Davis F, Chevalier N, Corra L, Czerucka D, Bottein MD, Demeneix B, Depledge M, Deheyn DD, Dorman CJ, Fénichel P, Fisher S, Gaill F, Galgani F, Gaze WH, Giuliano L, Grandjean P, Hahn ME, Hamdoun A, Hess P, Judson B, Laborde A, McGlade J, Mu J, Mustapha A, Neira M, Noble RT, Pedrotti ML, Reddy C, Rocklöv J, Scharler UM, Shanmugam H, Taghian G, van de Water JAJM, Vezzulli L, Weihe P, Zeka A, Raps H, Rampal P. Human Health and Ocean Pollution. Ann Glob Health. 2020 Dec 3;86(1):151. PMID:33354517 doi:10.5334/aogh.2831
- ↑ Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL. Marine pollution. Plastic waste inputs from land into the ocean. Science. 2015 Feb 13;347(6223):768-71. PMID:25678662 doi:10.1126/science.1260352
Student Contributors
Ashley Callaghan, Rebecca Hoff, & Simone McCowan