We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Carson Powers/Sandbox 1
From Proteopedia
< User:Carson Powers(Difference between revisions)
| (3 intermediate revisions not shown.) | |||
| Line 3: | Line 3: | ||
=Introduction= | =Introduction= | ||
| - | SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) is an RNA virus that is responsible for the highly infectious respiratory disease, COVID-19. It first emerged late in 2019 and quickly spread around the world | + | SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) is an RNA virus that is responsible for the highly infectious respiratory disease, COVID-19. It first emerged late in 2019 and quickly spread around the world. It earned the rank of "pandemic" by March of the following year and has resulted in the deaths of over seven million people.<ref name="WHO">World Health Organization. “COVID-19 Deaths | WHO COVID-19 Dashboard.” World Health Organization Data, 2024, data.who.int/dashboards/covid19/deaths</ref> Given the severity of the situation the entire world was in, researchers around the globe began researching the virus and methods to prevent its spread. One such method looked to stop the initial infection by preventing the endocytosis of the virus itself through direct competition (for the virus) with the receptor to which it binds. Given this is a respiratory infection, the mode by which to give this competitive inhibitor was designed to be a nose-spray. Initial research looked into the use of antibodies to accomplish this task but found antibodies were not stable enough for this mode. But the use of a new tool changed the game. Using a protein-predicting software, generated proteins, known as miniproteins or mini binders due to their relatively small size, solved the stability issue and greatly increased binding affinity. Not only has this tool led to a major step forward in the treatment of COVID-19, but it can even be applied to other infections, marking a new era in the development of therapeutics. |
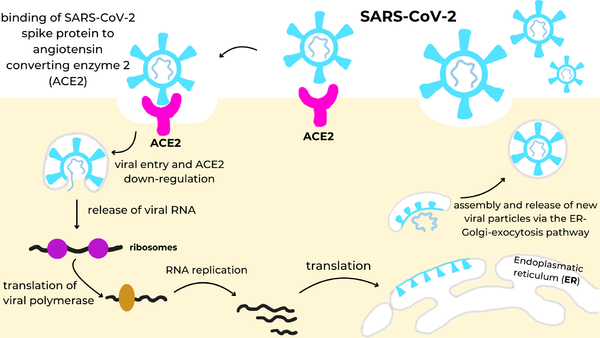
[[Image:Infection_Cycle.png|600px|right|SARS-CoV-2 infection cycle: the viral spike protein (cyan) binds to the ACE2 receptor (magenta) on the cell surface, triggering endocytosis and ACE2 down-regulation, release of viral RNA into the cytosol, translation of the viral polymerase and genome replication, synthesis of structural proteins in the endoplasmic reticulum (ER), and assembly and release of new virions via the ER-Golgi-exocytosis pathway.]] | [[Image:Infection_Cycle.png|600px|right|SARS-CoV-2 infection cycle: the viral spike protein (cyan) binds to the ACE2 receptor (magenta) on the cell surface, triggering endocytosis and ACE2 down-regulation, release of viral RNA into the cytosol, translation of the viral polymerase and genome replication, synthesis of structural proteins in the endoplasmic reticulum (ER), and assembly and release of new virions via the ER-Golgi-exocytosis pathway.]] | ||
| Line 25: | Line 25: | ||
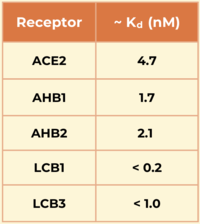
Another miniprotein, <scene name='10/1076041/Lcb3_scene_1/4'>LCB3</scene>, was developed using the same computational method as LCB1 and binds to the same region of the RBD, except in the opposite direction. Its <scene name='10/1076041/Lcb3xspikecorlabel_intxn/1'>binding interactions</scene> also show a large number of hydrogen bonds. However, LCB1 has more surface area and fits more precisely with the spike protein, giving it a slightly higher binding affinity (shown in the table to the right).[[Image:Kd_table.png|200px|right|'''Table 1'''. Dissociation constants (Kd) in nM for SARS-CoV-2 spike RBD binding to ACE2 and engineered minibinders, measured by biolayer interferometry to compare affinities for the spike protein.]] | Another miniprotein, <scene name='10/1076041/Lcb3_scene_1/4'>LCB3</scene>, was developed using the same computational method as LCB1 and binds to the same region of the RBD, except in the opposite direction. Its <scene name='10/1076041/Lcb3xspikecorlabel_intxn/1'>binding interactions</scene> also show a large number of hydrogen bonds. However, LCB1 has more surface area and fits more precisely with the spike protein, giving it a slightly higher binding affinity (shown in the table to the right).[[Image:Kd_table.png|200px|right|'''Table 1'''. Dissociation constants (Kd) in nM for SARS-CoV-2 spike RBD binding to ACE2 and engineered minibinders, measured by biolayer interferometry to compare affinities for the spike protein.]] | ||
| - | + | =Significance= | |
| - | The researchers demonstrated the advantages of using a de novo approach to design efficient miniprotein inhibitors that bind the SARS-CoV-2 spike protein with exceptionally high affinity. The dissociation constants (Kd) in nM for SARS-CoV-2 spike | + | The researchers demonstrated the advantages of using a de novo approach to design efficient miniprotein inhibitors that bind the SARS-CoV-2 spike protein with exceptionally high affinity. The dissociation constants (Kd) in nM for SARS-CoV-2 spike protein binding to the different receptors are shown in the table to the right. The small, hyperstable miniproteins that were created using this approach (i.e., LCB1 and LCB3) are smaller, more stable, cheaper to produce, and better suited for delivery (e.g., nasal sprays) compared to traditional antibodies. By targeting the receptor-binding domain (RBD) and competing directly with ACE2, these inhibitors block viral entry into host cells, as they bind over 50 times more tightly than the ACE2 receptor. The final structures of LCB1 and LCB3 differed by around had very low deviation (~ 1.3-1.9 Å) from their computer models, demonstrating the accuracy of the computational tools used in predicting the three-dimensional folding of the miniproteins along with their specific interactions with the spike RBD. |
| - | == | + | ==''Therapeutic Relevance''== |
| - | The LCBs have a significantly higher affinity for the spike RBD and are smaller and more stable than traditional antibodies. Their reduced size allows these molecules to tightly pack more interactions in the active site as well as improves their ability to enter the respiratory system. Their hyperstability makes them able to withstand high and low temperatures, allowing them to be stored anywhere--contributing to their higher efficacy than most traditional antibodies.<ref name="Cao"/> However, further research is needed as they have not used the miniproteins in animal studies or human trials yet, and there is a small risk of immunogenicity. | + | The LCBs have a significantly higher affinity for the spike RBD and are smaller and more stable than traditional antibodies. Their reduced size allows these molecules to tightly pack more interactions in the active site as well as improves their ability to enter the respiratory system. Their hyperstability makes them able to withstand high and low temperatures, allowing them to be stored anywhere--contributing to their higher efficacy than most traditional antibodies.<ref name="Cao"/> However, further research is needed as they have not used the miniproteins in animal studies or human trials yet, and there is a small risk of immunogenicity. Another flaw in this method is it assumes no mutations occur. Since viruses mutate very quickly, by the time these therapeutics are distributed, they may be rendered useless due to a slight change in the spike protein structure. But overall, the development of LCB1 and LCB3 validates the reliability, efficiency, and accuracy of the computational design approach, demonstrating a quick and efficient strategy for producing therapeutics in response to emerging infectious diseases. |
| - | + | =References= | |
<references/> | <references/> | ||
Current revision
De Novo Miniprotein COVID-19 Therapeutic
| |||||||||||