Sandbox Reserved 1846
From Proteopedia
(Difference between revisions)
| (4 intermediate revisions not shown.) | |||
| Line 39: | Line 39: | ||
=== Phe243 === | === Phe243 === | ||
| - | <scene name='10/1075247/F243_original/1'>Phe243</scene> is located 3.6 Å | + | <scene name='10/1075247/F243_original/1'>Phe243</scene> is located 3.6 Å <scene name='10/1075247/Original_15_mutation_structure/6'>from the ligand</scene>. Two mutations at this position, <scene name='10/1075247/F243i/1'>F243I</scene> and F243W, increase the catalytic activity of the enzyme. The larger tryptophan at position 243 stabilizes the ligand through stronger hydrophobic and π–π interactions, pulling it closer to the catalytic site despite its size. The F243I mutation inserts the smaller isoleucine whose side chain allows the ligand to sit closer. This reduces the ligand distance to 3.0 Å, improving substrate binding. The F243W mutation inserts the bulkier, nitrogen-containing aromatic aide chain. Trp brings the ligand slightly closer at 3.2 Å and introduces potential for new interactions, such as hydrogen bonding or [https://en.wikipedia.org/wiki/Pi-stacking#:~:text=In%20chemistry%2C%20pi%20stacking%20(also,interaction%22)%20is%20electrostatically%20repulsive. π-stacking]. Both mutations result in improved catalytic performance. The F243I mutant shows a 27.5% increase in activity, while the F243W mutant shows a 17.5% increase, compared to the wild-type enzyme.<ref name="Tournier"/> |
=== Tyr127 === | === Tyr127 === | ||
| - | The mutation of Tyr to Gly at <scene name='10/1075247/Y127/4'>Tyr127</scene> | + | The mutation of Tyr to Gly at <scene name='10/1075247/Y127/4'>Tyr127</scene>, which is <scene name='10/1075247/Original_15_mutation_structure/6'>adjacent to the hydrophobic groove</scene>, also increases the thermostability of LCC. The melting point of Y127G is increased to 87.0°C from the WT melting point of 84.7°C. Tyr has a bulky, rigid aromatic side chain that can cause structural strain, shown in <scene name='10/1075247/Y127_spacefill/1'>Y127 representation</scene>. Gly is the smallest amino acid and lacks a side chain, providing greater flexibility. The mutation <scene name='10/1075247/Y127g/2'>Y127G</scene> melting point is increased to 87.0°C. The mutation reduces [https://en.wikipedia.org/wiki/Steric_effects#:~:text=Steric%20hindrance%20is%20the%20slowing,as%20slowing%20unwanted%20side%2Dreactions. steric hindrance] and relieves strain in the protein structure, as demonstrated in the <scene name='10/1075247/Y127g_space_fill/1'>Y127G surface representation</scene>. By increasing flexibility, the Y127G mutation helps the protein maintain its folded structure under heat stress.<ref name="Tournier"/> |
=== Ser283 & Asp238 === | === Ser283 & Asp238 === | ||
| Line 54: | Line 54: | ||
The WCCG variant (F243W/D238C/S283C/Y127G) had specific activity slightly lower than ICCG, but showed even greater thermostability, with a melting temperature increase of +10.1°C. It reached 90% PET depolymerization in 10.5 hours at 72°C.<ref name="Tournier"/> | The WCCG variant (F243W/D238C/S283C/Y127G) had specific activity slightly lower than ICCG, but showed even greater thermostability, with a melting temperature increase of +10.1°C. It reached 90% PET depolymerization in 10.5 hours at 72°C.<ref name="Tournier"/> | ||
| - | Other stabilizing mutations, such as T96M, N246D, and N246M, were also tested, but excluded as they were not part of the top-performing mutant (ICCG) | + | Other stabilizing mutations, such as T96M, N246D, and N246M, were also tested, but excluded as they were not part of the top-performing multi-mutant variant (ICCG).<ref name="Tournier"/> |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
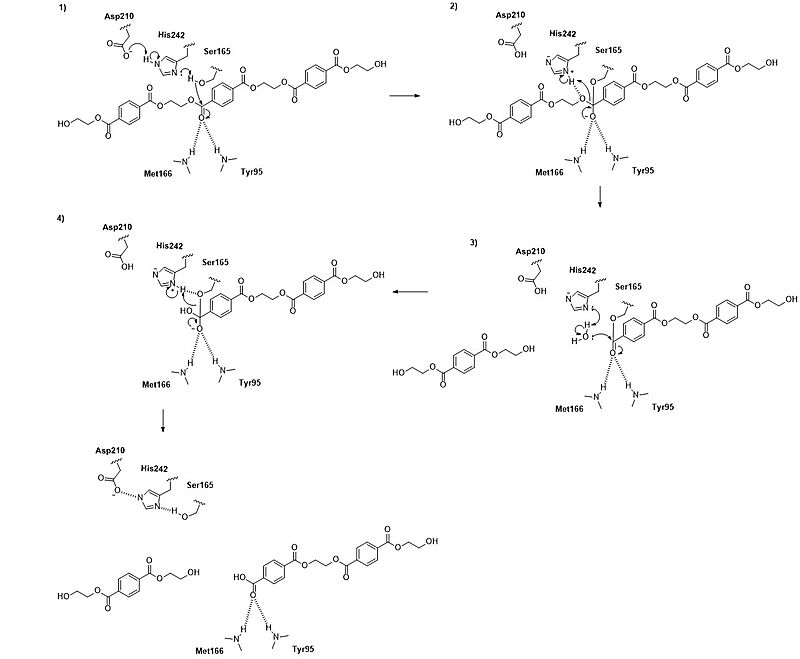
| - | A binding model of the substrate 2-HE(MHET)3 in wild-type | + | A binding model of the substrate 2-HE(MHET)3 in wild-type LCC (4eb0.pdb) was constructed and refined to mimic the 3D structure illustrated in Figure 2 of reference <ref name="Tournier"/>. The software Maestro (Schrödinger, Inc; version 14.2.118) was used to construct the initial binding structure, followed by energy minimization in the context of the rigid protein that had previously been processed to add/refine all hydrogen atoms. The ligand model was then used without further modification to identify and illustrate the cited active-site residues.<references/> |
== Student Contributors == | == Student Contributors == | ||
Ashley Callaghan, Rebecca Hoff, & Simone McCowan | Ashley Callaghan, Rebecca Hoff, & Simone McCowan | ||
Current revision
Leaf Branch Compost Cutinase
| |||||||||||
References
A binding model of the substrate 2-HE(MHET)3 in wild-type LCC (4eb0.pdb) was constructed and refined to mimic the 3D structure illustrated in Figure 2 of reference [1]. The software Maestro (Schrödinger, Inc; version 14.2.118) was used to construct the initial binding structure, followed by energy minimization in the context of the rigid protein that had previously been processed to add/refine all hydrogen atoms. The ligand model was then used without further modification to identify and illustrate the cited active-site residues.- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Tournier V, Topham CM, Gilles A, David B, Folgoas C, Moya-Leclair E, Kamionka E, Desrousseaux ML, Texier H, Gavalda S, Cot M, Guemard E, Dalibey M, Nomme J, Cioci G, Barbe S, Chateau M, Andre I, Duquesne S, Marty A. An engineered PET depolymerase to break down and recycle plastic bottles. Nature. 2020 Apr;580(7802):216-219. doi: 10.1038/s41586-020-2149-4. Epub 2020 Apr, 8. PMID:32269349 doi:http://dx.doi.org/10.1038/s41586-020-2149-4
- ↑ 2.0 2.1 2.2 2.3 2.4 Sui B, Wang T, Fang J, Hou Z, Shu T, Lu Z, Liu F, Zhu Y. Recent advances in the biodegradation of polyethylene terephthalate with cutinase-like enzymes. Front Microbiol. 2023 Oct 2;14:1265139. PMID:37849919 doi:10.3389/fmicb.2023.1265139
- ↑ Lichtenthaler HK. The stress concept in plants: an introduction. Ann N Y Acad Sci. 1998 Jun 30;851:187-98. PMID:9668620 doi:10.1111/j.1749-6632.1998.tb08993.x
- ↑ Ueda H, Tabata J, Seshime Y, Masaki K, Sameshima-Yamashita Y, Kitamoto H. Cutinase-like biodegradable plastic-degrading enzymes from phylloplane yeasts have cutinase activity. Biosci Biotechnol Biochem. 2021 Jul 23;85(8):1890-1898. PMID:34160605 doi:10.1093/bbb/zbab113
- ↑ Kolattukudy PE. Biopolyester membranes of plants: cutin and suberin. Science. 1980 May 30;208(4447):990-1000. PMID:17779010 doi:10.1126/science.208.4447.990
- ↑ 6.0 6.1 6.2 6.3 6.4 Khairul Anuar NFS, Huyop F, Ur-Rehman G, Abdullah F, Normi YM, Sabullah MK, Abdul Wahab R. An Overview into Polyethylene Terephthalate (PET) Hydrolases and Efforts in Tailoring Enzymes for Improved Plastic Degradation. Int J Mol Sci. 2022 Oct 20;23(20):12644. PMID:36293501 doi:10.3390/ijms232012644
- ↑ 7.0 7.1 Burgin T, Pollard BC, Knott BC, Mayes HB, Crowley MF, McGeehan JE, Beckham GT, Woodcock HL. The reaction mechanism of the Ideonella sakaiensis PETase enzyme. Commun Chem. 2024 Mar 27;7(1):65. PMID:38538850 doi:10.1038/s42004-024-01154-x
- ↑ 8.0 8.1 8.2 Zhang J, Wang H, Luo Z, Yang Z, Zhang Z, Wang P, Li M, Zhang Y, Feng Y, Lu D, Zhu Y. Computational design of highly efficient thermostable MHET hydrolases and dual enzyme system for PET recycling. Commun Biol. 2023 Nov 9;6(1):1135. PMID:37945666 doi:10.1038/s42003-023-05523-5
- ↑ Yoshida S, Hiraga K, Takehana T, Taniguchi I, Yamaji H, Maeda Y, Toyohara K, Miyamoto K, Kimura Y, Oda K. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science. 2016 Mar 11;351(6278):1196-9. doi: 10.1126/science.aad6359. PMID:26965627 doi:http://dx.doi.org/10.1126/science.aad6359
- ↑ Landrigan PJ, Stegeman JJ, Fleming LE, Allemand D, Anderson DM, Backer LC, Brucker-Davis F, Chevalier N, Corra L, Czerucka D, Bottein MD, Demeneix B, Depledge M, Deheyn DD, Dorman CJ, Fénichel P, Fisher S, Gaill F, Galgani F, Gaze WH, Giuliano L, Grandjean P, Hahn ME, Hamdoun A, Hess P, Judson B, Laborde A, McGlade J, Mu J, Mustapha A, Neira M, Noble RT, Pedrotti ML, Reddy C, Rocklöv J, Scharler UM, Shanmugam H, Taghian G, van de Water JAJM, Vezzulli L, Weihe P, Zeka A, Raps H, Rampal P. Human Health and Ocean Pollution. Ann Glob Health. 2020 Dec 3;86(1):151. PMID:33354517 doi:10.5334/aogh.2831
- ↑ Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL. Marine pollution. Plastic waste inputs from land into the ocean. Science. 2015 Feb 13;347(6223):768-71. PMID:25678662 doi:10.1126/science.1260352
- ↑ Austin HP, Allen MD, Donohoe BS, Rorrer NA, Kearns FL, Silveira RL, Pollard BC, Dominick G, Duman R, El Omari K, Mykhaylyk V, Wagner A, Michener WE, Amore A, Skaf MS, Crowley MF, Thorne AW, Johnson CW, Woodcock HL, McGeehan JE, Beckham GT. Characterization and engineering of a plastic-degrading aromatic polyesterase. Proc Natl Acad Sci U S A. 2018 Apr 17. pii: 1718804115. doi:, 10.1073/pnas.1718804115. PMID:29666242 doi:http://dx.doi.org/10.1073/pnas.1718804115
Student Contributors
Ashley Callaghan, Rebecca Hoff, & Simone McCowan