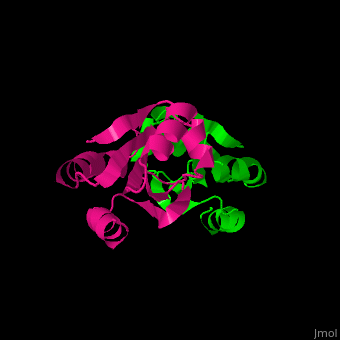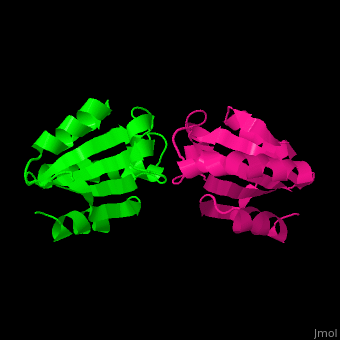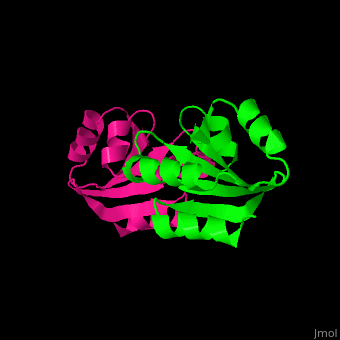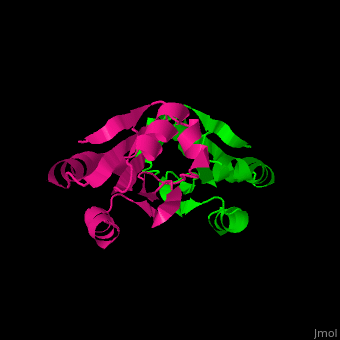Thioredoxin
From Proteopedia
(Difference between revisions)
| (9 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
<StructureSection load='' size='350' side='right' caption='Human thioredoxin (PDB entry [[1ert]])' scene='43/430885/Cv/2'> | <StructureSection load='' size='350' side='right' caption='Human thioredoxin (PDB entry [[1ert]])' scene='43/430885/Cv/2'> | ||
| - | == Function == | + | == Function == |
In response to the toxicity of reactive oxygen species (ROS), pathogenic organisms such as fungi possess enzymatic defense mechanisms. One such enzyme is Thioredoxin Reductase (TrxR), which plays essential roles in several biological processes including DNA synthesis, redox signaling, and oxidative stress response. Inhibiting TrxR can weaken pathogens, which may have significant implications for public health and agriculture. | In response to the toxicity of reactive oxygen species (ROS), pathogenic organisms such as fungi possess enzymatic defense mechanisms. One such enzyme is Thioredoxin Reductase (TrxR), which plays essential roles in several biological processes including DNA synthesis, redox signaling, and oxidative stress response. Inhibiting TrxR can weaken pathogens, which may have significant implications for public health and agriculture. | ||
| - | Notably, fungal TrxRs are structurally and biochemically distinct from those found in mammals — a desirable feature for drug development efforts | + | Notably, fungal TrxRs are structurally and biochemically distinct from those found in mammals — a desirable feature for drug development efforts<ref>Oliveira LO. (2010). Caracterização de tiorredoxinas de fungos filamentosos como alvos moleculares para drogas antifúngicas. Tese de doutorado, Universidade de São Paulo.</ref>. Furthermore, fungal TrxRs do not reduce the host thioredoxin, supporting their relevance as selective drug targets. |
=== The Thioredoxin System === | === The Thioredoxin System === | ||
The thioredoxin system is composed of two main proteins: Thioredoxin (Trx) and Thioredoxin Reductase (TrxR), along with NADPH as a cofactor. This system functions primarily by reducing antioxidant proteins such as Peroxiredoxins (Prx) and Methionine Sulfoxide Reductases. It is also involved in other redox regulatory functions. | The thioredoxin system is composed of two main proteins: Thioredoxin (Trx) and Thioredoxin Reductase (TrxR), along with NADPH as a cofactor. This system functions primarily by reducing antioxidant proteins such as Peroxiredoxins (Prx) and Methionine Sulfoxide Reductases. It is also involved in other redox regulatory functions. | ||
| - | Historically, Trxs were the first enzymes identified as responsible for reducing ribonucleotides to deoxyribonucleotides in *Escherichia coli*, an essential step in DNA synthesis | + | Historically, Trxs were the first enzymes identified as responsible for reducing ribonucleotides to deoxyribonucleotides in *Escherichia coli*, an essential step in DNA synthesis<ref>Laurent TC, Moore EC, Reichard P. (1964). J Biol Chem. 239(10):3436–3444.</ref>. |
=== Mechanism of Action === | === Mechanism of Action === | ||
| Line 19: | Line 19: | ||
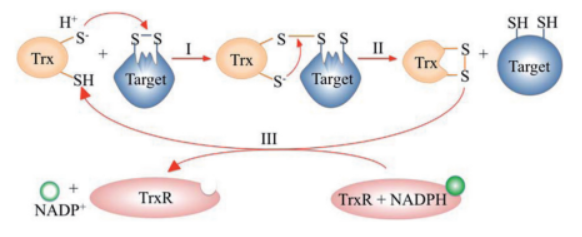
* **Step I:** The N-terminal cysteine of Trx attacks a disulfide bond in the target protein, forming a mixed disulfide intermediate. | * **Step I:** The N-terminal cysteine of Trx attacks a disulfide bond in the target protein, forming a mixed disulfide intermediate. | ||
* **Step II:** The C-terminal cysteine of Trx resolves the intermediate, releasing the reduced protein and generating oxidized Trx. | * **Step II:** The C-terminal cysteine of Trx resolves the intermediate, releasing the reduced protein and generating oxidized Trx. | ||
| - | * **Step III:** TrxR recycles Trx using electrons from NADPH | + | * **Step III:** TrxR recycles Trx using electrons from NADPH<ref>Netto LES et al. (2015). Free Radic Biol Med. 89:60–75.</ref>. |
[[Image:Thioredoxin_cycle.png|600px]] | [[Image:Thioredoxin_cycle.png|600px]] | ||
| - | ''Figure 1: Catalytic cycle of the thioredoxin system. Adapted from Netto et al., 2015.'' | ||
| + | ''Figure 1: Catalytic cycle of the thioredoxin system. Adapted from Netto et al., 2015.'' | ||
| - | + | [[Thioredoxin]] (Trx) is an enzyme which facilitates, in its reduced form, the reduction of proteins by cysteine thiol-disulfide exchange<ref>Holmgren A. (2000). Antioxid Redox Signal. 2(4):811–820.</ref>. They contain a CXXC motif.<br /> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | [[Thioredoxin]] (Trx) is an enzyme which facilitates, in its reduced form, | + | |
* '''Trx-1''' is a mammalian cellular protein.<br /> | * '''Trx-1''' is a mammalian cellular protein.<br /> | ||
| - | * | + | * '''Trx-2''' is mitochondria-specific.<br /> |
| - | * | + | * '''Trx C,M,X,Y''' are found in prokaryotes.<br /> |
| - | * | + | * '''Trx F,H,O''' are found in eukaryotes.<br /> |
| - | + | ||
| + | There are a range of strategies used by the host organism in an attempt to defend itself against pathogen invasion. Among these is the release of oxidants, such as reactive oxygen species (ROS), which at defined physiological concentrations act as signaling messengers. However, in supraphysiological concentrations, their reactivity has deleterious cellular consequences, causing damage to macromolecules such as proteins, lipids and DNA, thus impairing the system's homeostasis<ref>Sies H. (1985). Oxidative stress: introductory remarks. Academic Press.</ref>. It is therefore an alternative used to inhibit the pathogen and prevent infection of the organism. | ||
== Relevance == | == Relevance == | ||
| - | Serum Trx level is a predictor of steatohepatitis<ref> | + | Serum Trx level is a predictor of steatohepatitis<ref>Yamada G et al. (2003). J Hepatol. 38(1):32–38.</ref>. |
== Disease == | == Disease == | ||
| - | Trx is involved in a wide range of human diseases and conditions including cancer, viral diseases, aging, cardiac conditions and more<ref> | + | Trx is involved in a wide range of human diseases and conditions including cancer, viral diseases, aging, cardiac conditions and more<ref>Arnér ESJ, Holmgren A. (2006). Semin Cancer Biol. 16(6):420–426.</ref>. |
== Structural highlights == | == Structural highlights == | ||
| - | The <scene name='43/430885/Cv/4'>active site motif Cys-Gly-Pro-Cys</scene> is involved in the reduction of disulfide bonds in proteins<ref> | + | |
| + | The <scene name='43/430885/Cv/4'>active site motif Cys-Gly-Pro-Cys</scene> is involved in the reduction of disulfide bonds in proteins<ref>Åslund F et al. (1997). J Biol Chem. 272(48):30780–30786.</ref>. | ||
| + | Unlike many other thioredoxins, the human cytoplasmic thioredoxin has three cysteine residues (Cys 62, Cys 69, Cys 73) additional to the active site <scene name='43/430885/Cv/2'>Cys 32 and Cys 35</scene>. | ||
| + | The human cytoplasmic thioredoxin crystal structure reveals a homodimer with <scene name='43/430885/Cv/2'>Cys 73 forming an intermolecular disulfide bridge</scene> | ||
== 3D Structures of Thioredoxin == | == 3D Structures of Thioredoxin == | ||
| Line 57: | Line 52: | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | |||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Oliveira LO. (2010). Caracterização de tiorredoxinas de fungos filamentosos como alvos moleculares para drogas antifúngicas. Tese de doutorado, Universidade de São Paulo.
- ↑ Laurent TC, Moore EC, Reichard P. (1964). J Biol Chem. 239(10):3436–3444.
- ↑ Netto LES et al. (2015). Free Radic Biol Med. 89:60–75.
- ↑ Holmgren A. (2000). Antioxid Redox Signal. 2(4):811–820.
- ↑ Sies H. (1985). Oxidative stress: introductory remarks. Academic Press.
- ↑ Yamada G et al. (2003). J Hepatol. 38(1):32–38.
- ↑ Arnér ESJ, Holmgren A. (2006). Semin Cancer Biol. 16(6):420–426.
- ↑ Åslund F et al. (1997). J Biol Chem. 272(48):30780–30786.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Laura Maria Batista Leal, Joel L. Sussman