TRPC1 TRPC4 8WPL BI3323 Aug2025
From Proteopedia
(Difference between revisions)
m (Sandbox TRPC1 TRPC4 8WPL moved to TRPC1 TRPC4 8WPL BI3323 Aug2025) |
(final changes) |
||
| (8 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
==Overview of the TRPC1/TRPC4 Channel== | ==Overview of the TRPC1/TRPC4 Channel== | ||
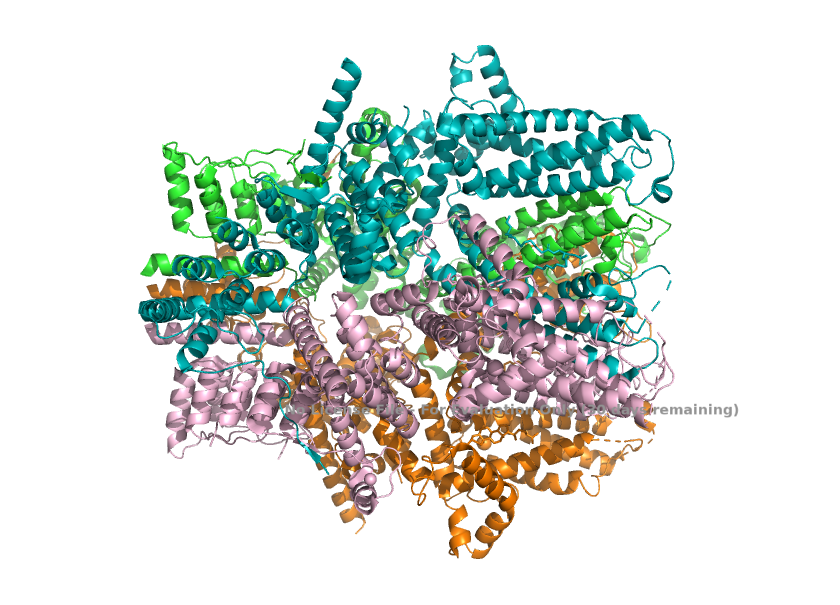
<StructureSection load='8WPL' size='340' side='right' caption='Cryo-EM structure of the heteromeric human TRPC1/TRPC4 channel (PDB: 8WPL)' scene=''> | <StructureSection load='8WPL' size='340' side='right' caption='Cryo-EM structure of the heteromeric human TRPC1/TRPC4 channel (PDB: 8WPL)' scene=''> | ||
| - | This is a | + | This paper shows the detailed 3D structure of the human TRPC1-TRPC4 ion channel. This channel is made of one TRPC1 nd three TRPC4 subunits. The researchers captured(using cro-Electron Microscopy) what the channel looks like normally and when blocked by a drug, Pico145. <ref>Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. ''Nature Structural & Molecular Biology'', 32(2):326–338. DOI: 10.1038/s41594-024-01408-1</ref> |
| - | + | Main finding: The incorporation of one TRPC1 completely changes the shape of the TRPC4 channel. It changes TRPC4 from a perfectly symmetrical pore to an asymmetric heteromer(TRPC1/TRPC4). This asymmetry narrows the ion pathway, bends several helices, and creates a different lower gate. This decreases the calcium permeability and shifts the preference to monovalent ions such as sodium and potassium. Electrical tests and mutant experiments confirm that this structural shift affects how the channel actually works.<ref>Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. ''Nature Structural & Molecular Biology'', 32(2):326–338. DOI: 10.1038/s41594-024-01408-1</ref> | |
| + | The structure in this paper also shows why the drug Pico145 binds tightly. This is because TRPC1 provides extra hydrophobic residues( F575 and F592) that form a tighter drug-binding pocket, which makes Pico145 more effective on TRPC1/TRPC4 channels compared to TRPC-only channels.<ref>Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. ''Nature Structural & Molecular Biology'', 32(2):326–338. DOI: 10.1038/s41594-024-01408-1</ref> | ||
| + | Overall, these structural insights | ||
== Function == 1.TRCP1 helps the cell to fill up for SERCA2 deficiency so that the cell can survive by letting more calcium enter the endoplasmic reticulum. This activates survival pathways, such as NF-kB, reduces cell death, and promotes cell growth.<ref>Pani, B., Cornatzer, E. et al. (2006). Up-Regulation of Transient Receptor Potential Canonical 1 (TRPC1) following Sarco(endo)plasmic Reticulum Ca²⁺ ATPase 2 Gene Silencing Promotes Cell Survival: A Potential Role for TRPC1 in Darier's Disease. ''Molecular Biology of the Cell'', 17(10):4446–4458.</ref> | == Function == 1.TRCP1 helps the cell to fill up for SERCA2 deficiency so that the cell can survive by letting more calcium enter the endoplasmic reticulum. This activates survival pathways, such as NF-kB, reduces cell death, and promotes cell growth.<ref>Pani, B., Cornatzer, E. et al. (2006). Up-Regulation of Transient Receptor Potential Canonical 1 (TRPC1) following Sarco(endo)plasmic Reticulum Ca²⁺ ATPase 2 Gene Silencing Promotes Cell Survival: A Potential Role for TRPC1 in Darier's Disease. ''Molecular Biology of the Cell'', 17(10):4446–4458.</ref> | ||
2) when TRPC1 joins TRPC4 channel (1 TRPC1 and TRPC4) it pushes away Ca²⁺ as TRPC1 has an aminio acid(K639) which is positively charged pushing the positively charged calcium. It also develops more preference for Na⁺/K⁺ over Ca²⁺, while increasing inhibitor sensitivity.<ref>Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. ''Nature Structural & Molecular Biology'', 32(2):326–338. DOI: 10.1038/s41594-024-01408-1</ref> | 2) when TRPC1 joins TRPC4 channel (1 TRPC1 and TRPC4) it pushes away Ca²⁺ as TRPC1 has an aminio acid(K639) which is positively charged pushing the positively charged calcium. It also develops more preference for Na⁺/K⁺ over Ca²⁺, while increasing inhibitor sensitivity.<ref>Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. ''Nature Structural & Molecular Biology'', 32(2):326–338. DOI: 10.1038/s41594-024-01408-1</ref> | ||
3. TRPC4 is essential during early postnatal brain development, as it helps dendrites grow and stay stable by turning glutamate signals into calcium entry, activating a force-generating pathway. Without TRPC4, dendrites will disintegrate.<ref>Jeon, J., Moore, T. I., Sob, I. et al. (2025). TRPC4 regulates limbic behavior and neuronal development by stabilizing dendrite branches through actomyosin-driven integrin activation. ''PNAS'', 122(33):e2511037ca122.</ref> | 3. TRPC4 is essential during early postnatal brain development, as it helps dendrites grow and stay stable by turning glutamate signals into calcium entry, activating a force-generating pathway. Without TRPC4, dendrites will disintegrate.<ref>Jeon, J., Moore, T. I., Sob, I. et al. (2025). TRPC4 regulates limbic behavior and neuronal development by stabilizing dendrite branches through actomyosin-driven integrin activation. ''PNAS'', 122(33):e2511037ca122.</ref> | ||
| - | == Disease == | + | == Disease == 1. Darier's disease, which is a genetic disorder, is caused by a mutation in SERCA2, the pump that stores calcium inside the endoplasmic reticulum. This inhibits Calcium flow inside, causing upregulation of TRPC1 to allow more calcium to enter. However, this activates NF-kB survival pathway, resists cell death, and pushes them towards overgrowth or abnormal keratinization.<ref>Pani, B., Cornatzer, E. et al. (2006). Up-Regulation of Transient Receptor Potential Canonical 1 (TRPC1) following Sarco(endo)plasmic Reticulum Ca²⁺ ATPase 2 Gene Silencing Promotes Cell Survival: A Potential Role for TRPC1 in Darier's Disease. ''Molecular Biology of the Cell'', 17(10):4446–4458.</ref> |
| + | 2. TRPC4 helps stabilize the dendritic branches. Without TRPC4, brain circuits form incorrectly and cause neurodevelopmental effects.<ref>Jeon, J., Moore, T. I., Sob, I. et al. (2025). TRPC4 regulates limbic behavior and neuronal development by stabilizing dendrite branches through actomyosin-driven integrin activation. ''PNAS'', 122(33):e2511037122.</ref> | ||
| - | == Relevance == | + | == Relevance ==1. The asymmetric structure of TRPC1:TRPC4 creates a distinct identity compared to TRPC4 homomers. This would help Pharmaceutical scientists to develop drugs that specifically target the TRPC1/TRPC4 heteromer without affecting TRPC4 or other TRP family channels.<ref>Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. ''Nature Structural & Molecular Biology'', 32(2):326–338. DOI: 10.1038/s41594-024-01408-1</ref> |
| + | 2. The structure shows how the antagonist Pico145 binds to the channel, creating a stronger hydrophobic interaction than TRPC4-only channels.<ref>Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. ''Nature Structural & Molecular Biology'', 32(2):326–338. DOI: 10.1038/s41594-024-01408-1</ref>. This can explain why Pico145 is more potent against TRPC1-containing channels or how drug binding is influenced by heteromer composition. This information can improve selectivity and reduce side effects. | ||
| + | 3. Residues in TRPC1, such as L601(selectivity filter) and K639(in the central cavity), explains how TRPC1 alters TRPC4's functions.<ref>Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. ''Nature Structural & Molecular Biology'', 32(2):326–338. DOI: 10.1038/s41594-024-01408-1</ref>. This helps us understand how ion permeability leads to diseases. | ||
| - | == Structural highlights == | + | |
| + | == Structural highlights ==1. The channel, when TRPC1 is incorporated, loses the 4-fold symmetry with one TRPC1 and three TRPC4. This change in arrangement breaks the symmetry of the pore and creates a different, asymmetric ion-conduction pathway. <ref>Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. ''Nature Structural & Molecular Biology'', 32(2):326–338. DOI: 10.1038/s41594-024-01408-1</ref> | ||
| + | |||
| + | [[Image:Asymmetry_(TRPC1_TRPC4_Stoichiometry)_.png]] | ||
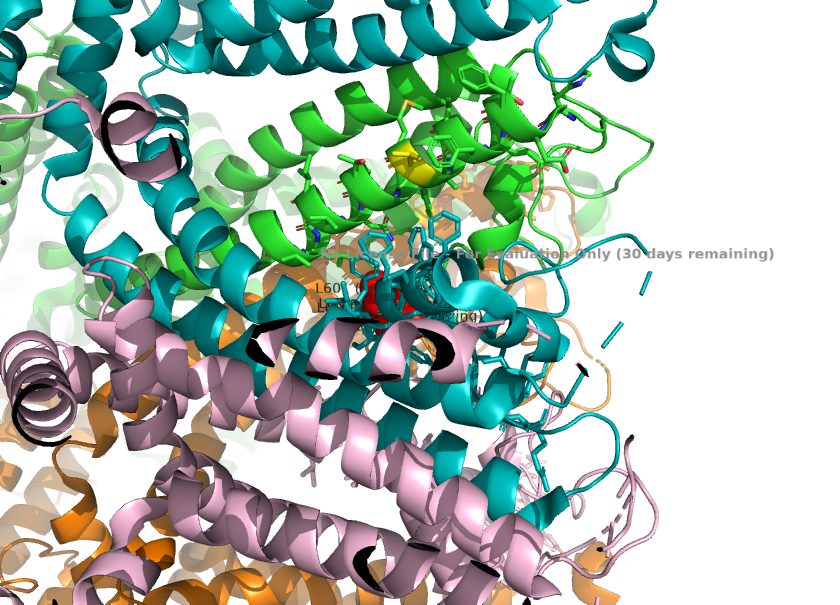
| + | 2. The selectivity filter loop(two maino acids longer than the corresponding loop in TRPC4) causes the loop to protrude further into the pore. L601 from TRPC1 is the key residue responsible for this, as it physically projects into the ion pathway, narrowing the pore radius. These change the channels' preference for monovalent channels. | ||
| + | <ref>Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. ''Nature Structural & Molecular Biology'', 32(2):326–338. DOI: 10.1038/s41594-024-01408-1</ref> | ||
| + | |||
| + | [[Image:Selectivity_Filter_Narrowing_(L601)_.png]] | ||
| + | |||
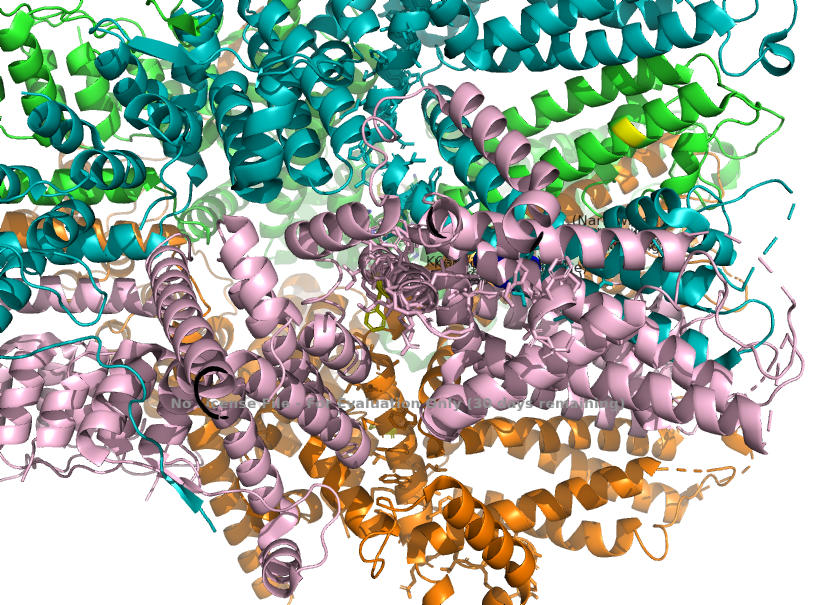
| + | 3. Calcium permeability is determined by the S6 helix present depeer in the pore. The TRPC1 subunit provides K639 here, which carries a positive charge in the central cavity of the pore. This creates an electropositive environment repelling calcium, which is also positively charged. <ref>Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. ''Nature Structural & Molecular Biology'', 32(2):326–338. DOI: 10.1038/s41594-024-01408-1</ref> | ||
| + | [[Image:Calcium_Permeability_(K639_in_S6)_.png]] | ||
Current revision
Overview of the TRPC1/TRPC4 Channel
| |||||||||||
References
- ↑ Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. Nature Structural & Molecular Biology, 32(2):326–338. DOI: 10.1038/s41594-024-01408-1
- ↑ Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. Nature Structural & Molecular Biology, 32(2):326–338. DOI: 10.1038/s41594-024-01408-1
- ↑ Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. Nature Structural & Molecular Biology, 32(2):326–338. DOI: 10.1038/s41594-024-01408-1
- ↑ Pani, B., Cornatzer, E. et al. (2006). Up-Regulation of Transient Receptor Potential Canonical 1 (TRPC1) following Sarco(endo)plasmic Reticulum Ca²⁺ ATPase 2 Gene Silencing Promotes Cell Survival: A Potential Role for TRPC1 in Darier's Disease. Molecular Biology of the Cell, 17(10):4446–4458.
- ↑ Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. Nature Structural & Molecular Biology, 32(2):326–338. DOI: 10.1038/s41594-024-01408-1
- ↑ Jeon, J., Moore, T. I., Sob, I. et al. (2025). TRPC4 regulates limbic behavior and neuronal development by stabilizing dendrite branches through actomyosin-driven integrin activation. PNAS, 122(33):e2511037ca122.
- ↑ Pani, B., Cornatzer, E. et al. (2006). Up-Regulation of Transient Receptor Potential Canonical 1 (TRPC1) following Sarco(endo)plasmic Reticulum Ca²⁺ ATPase 2 Gene Silencing Promotes Cell Survival: A Potential Role for TRPC1 in Darier's Disease. Molecular Biology of the Cell, 17(10):4446–4458.
- ↑ Jeon, J., Moore, T. I., Sob, I. et al. (2025). TRPC4 regulates limbic behavior and neuronal development by stabilizing dendrite branches through actomyosin-driven integrin activation. PNAS, 122(33):e2511037122.
- ↑ Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. Nature Structural & Molecular Biology, 32(2):326–338. DOI: 10.1038/s41594-024-01408-1
- ↑ Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. Nature Structural & Molecular Biology, 32(2):326–338. DOI: 10.1038/s41594-024-01408-1
- ↑ Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. Nature Structural & Molecular Biology, 32(2):326–338. DOI: 10.1038/s41594-024-01408-1
- ↑ Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. Nature Structural & Molecular Biology, 32(2):326–338. DOI: 10.1038/s41594-024-01408-1
- ↑ Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. Nature Structural & Molecular Biology, 32(2):326–338. DOI: 10.1038/s41594-024-01408-1
- ↑ Won, J., Kim, J., Kim, J. et al. (2025). Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel. Nature Structural & Molecular Biology, 32(2):326–338. DOI: 10.1038/s41594-024-01408-1