Kaushki Sharma- BI3323
From Proteopedia
(Difference between revisions)
| (One intermediate revision not shown.) | |||
| Line 28: | Line 28: | ||
==Structure Tour== | ==Structure Tour== | ||
<StructureSection load='9kkk' size='340' side='right'caption='Cryo-EM structure of human SLC22A6 (OAT1) in the apo-state, [[Resolution|resolution]] 3.85Å' scene=''> | <StructureSection load='9kkk' size='340' side='right'caption='Cryo-EM structure of human SLC22A6 (OAT1) in the apo-state, [[Resolution|resolution]] 3.85Å' scene=''> | ||
| - | |||
| - | ===Introduction=== | ||
| - | |||
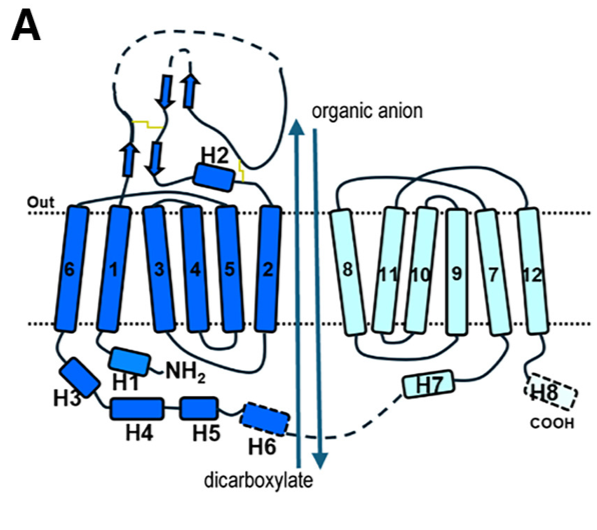
| - | Members of the organic anion transporter (OAT) family, including | ||
| - | OAT1, are expressed on the epithelial membrane of the kidney, | ||
| - | liver, brain, intestine, and placenta.<ref>Molecular cloning and characterization of a novel liver-specific transport protein https://doi.org/10.1242/jcs.107.4.1065</ref><ref>Molecular Cloning and Characterization of NKT, a Gene Product Related to the Organic Cation Transporter Family That Is Almost Exclusively Expressed in the Kidney https://doi.org/10.1074/jbc.272.10.6471</ref> OAT1 regulates the transport | ||
| - | of organic anion drugs from the blood into kidney epithelial | ||
| - | cells by utilizing the α-ketoglutarate (α-KG) gradient across the | ||
| - | membrane established by the tricarboxylic acid (TCA) cycle.<ref>Ingraham, L., Li, M., Renfro, J.L., Parker, S., Vapurcuyan, A., Hanna, I., and | ||
| - | Pelis, R.M. (2014). A plasma concentration of α-ketoglutarate influences | ||
| - | the kinetic interaction of ligands with organic anion transporter 1. Mol. | ||
| - | Pharmacol. 86, 86–95. https://doi.org/10.1124/mol.114.091777.</ref> <ref>Uwai, Y., Kawasaki, T., and Nabekura, T. (2017). D-Malate decreases renal | ||
| - | content of α-ketoglutarate, a driving force of organic anion transporters | ||
| - | OAT1 and OAT3, resulting in inhibited tubular secretion of phenolsulfonphthalein, | ||
| - | in rats. Biopharm. Drug Dispos. 38, 479–485. https://doi.org/10. | ||
| - | 1002/bdd.2089.</ref>OAT1 also plays a key role in excreting waste from organic drug metabolism and | ||
| - | contributes significantly to drug-drug interactions and drug disposition. However, the structural basis of specific | ||
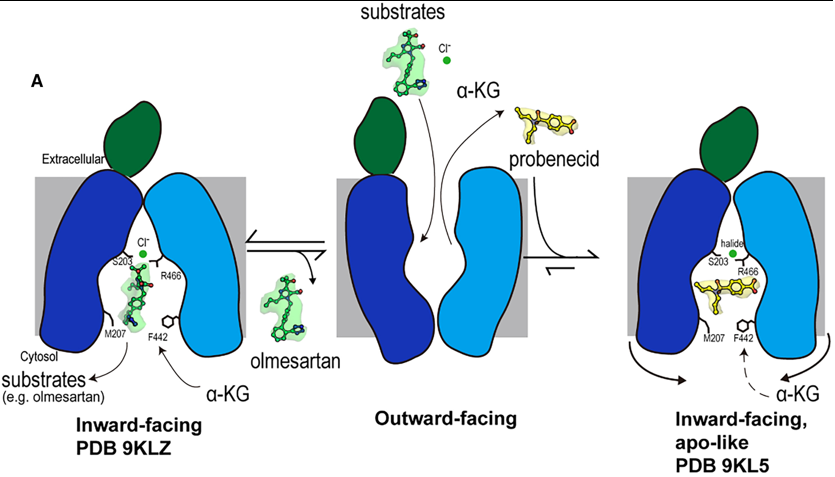
| - | substrate and inhibitor transport by human OAT1 (hOAT1) has remained elusive. Here are four | ||
| - | [[cryo-electron microscopy]] (cryo-EM) structures of hOAT1 in its inward-facing conformation: the apo | ||
| - | form, the substrate (olmesartan)-bound form with different anions, and the inhibitor (probenecid)-bound | ||
| - | form. | ||
| - | |||
Classification: MEMBRANE PROTEIN | Classification: MEMBRANE PROTEIN | ||
| Line 73: | Line 51: | ||
Reconstruction Method: SINGLE PARTICLE | Reconstruction Method: SINGLE PARTICLE | ||
| + | |||
| + | ===Introduction=== | ||
| + | |||
| + | Members of the organic anion transporter (OAT) family, including | ||
| + | OAT1, are expressed on the epithelial membrane of the kidney, | ||
| + | liver, brain, intestine, and placenta.<ref>Molecular cloning and characterization of a novel liver-specific transport protein https://doi.org/10.1242/jcs.107.4.1065</ref><ref>Molecular Cloning and Characterization of NKT, a Gene Product Related to the Organic Cation Transporter Family That Is Almost Exclusively Expressed in the Kidney https://doi.org/10.1074/jbc.272.10.6471</ref> OAT1 regulates the transport | ||
| + | of organic anion drugs from the blood into kidney epithelial | ||
| + | cells by utilizing the α-ketoglutarate (α-KG) gradient across the | ||
| + | membrane established by the tricarboxylic acid (TCA) cycle.<ref>Ingraham, L., Li, M., Renfro, J.L., Parker, S., Vapurcuyan, A., Hanna, I., and | ||
| + | Pelis, R.M. (2014). A plasma concentration of α-ketoglutarate influences | ||
| + | the kinetic interaction of ligands with organic anion transporter 1. Mol. | ||
| + | Pharmacol. 86, 86–95. https://doi.org/10.1124/mol.114.091777.</ref> <ref>Uwai, Y., Kawasaki, T., and Nabekura, T. (2017). D-Malate decreases renal | ||
| + | content of α-ketoglutarate, a driving force of organic anion transporters | ||
| + | OAT1 and OAT3, resulting in inhibited tubular secretion of phenolsulfonphthalein, | ||
| + | in rats. Biopharm. Drug Dispos. 38, 479–485. https://doi.org/10. | ||
| + | 1002/bdd.2089.</ref>OAT1 also plays a key role in excreting waste from organic drug metabolism and | ||
| + | contributes significantly to drug-drug interactions and drug disposition. However, the structural basis of specific | ||
| + | substrate and inhibitor transport by human OAT1 (hOAT1) has remained elusive. Here are four | ||
| + | [[cryo-electron microscopy]] (cryo-EM) structures of hOAT1 in its inward-facing conformation: the apo | ||
| + | form, the substrate (olmesartan)-bound form with different anions, and the inhibitor (probenecid)-bound | ||
| + | form. | ||
===Cryo-EM structure of hOAT1=== | ===Cryo-EM structure of hOAT1=== | ||
| Line 157: | Line 156: | ||
Molecular basis for selective uptake and elimination of organic anions in | Molecular basis for selective uptake and elimination of organic anions in | ||
the kidney by OAT1. Nat. Struct. Mol. Biol. 30, 1786–1793. https://doi. | the kidney by OAT1. Nat. Struct. Mol. Biol. 30, 1786–1793. https://doi. | ||
| - | org/10.1038/s41594-023-01039-y.</ref> and our hOAT1 structures align with findings for rOAT1 and provide new insights into the mechanism by which probenecid inhibits transport activity. Additionally, this study reveals the structure of hOAT1 with olmesartan, offering mechanistic insights into species-specific differences in OAT1 transport of specific substrates. | + | org/10.1038/s41594-023-01039-y.</ref> and our hOAT1 structures align with findings for rOAT1 and provide new insights into the mechanism by which probenecid inhibits transport activity. Additionally, this study reveals the structure of hOAT1 with olmesartan, offering mechanistic insights into species-specific differences in OAT1 transport of specific substrates. |
| + | This web page was created for an assignment in Course BI3323-Aug2025 (Structural Biology), IISER, Pune | ||
==References== | ==References== | ||
<references /> | <references /> | ||
Current revision
Interactive 3D Complement in Proteopedia
|
| |
|
Cryo-EM structures of human OAT1 reveal drug binding and inhibition mechanisms[1]. | |
|
Cell Volume 33, Issue 11, P1856-1866.E5, November 06, 2025 |
Structure Tour
| |||||||||||