|
|
| (412 intermediate revisions not shown.) |
| Line 1: |
Line 1: |
| - | [Article by Clifford Felder, Structural Biology, Weizmann Institute, 18 November 2008.] | + | <StructureSection load='' size='350' side='right' scene='22/22/Ache_with_ach/2' caption='Torpedo california AChE (PDB code [[2ace]])'> |
| | + | [[Image:small_wh_ray0001.gif|left|150px]]<br /> |
| | + | '''Acetylcholinesterase''' (AChE) is key enzyme in the nervous system of animals. By rapid hydrolysis of the neurotransmitter, [[Acetylcholine|acetylcholine]] (ACh), AChE terminates neurotransmission at cholinergic synapses. It is a very fast enzyme, especially for a serine hydrolase, functioning at a rate approaching that of a diffusion-controlled reaction. AChE inhibitors are among the key drugs approved by the FDA for management of Alzheimer's disease (AD). The powerful toxicity of organophosphorus (OP) poisons is attributed primarily to their potent AChE inhibitors. |
| | | | |
| - | THIS PAGE IS **UNDER CONSTRUCTION**, PLEASE BE PATIENT UNTIL IT IS COMPLETED!
| + | See also [[Acetylcholinesterase (Hebrew)]] |
| | | | |
| - | The increasing longevity of people's lifespans, and the resulting increased
| + | == Key Enzyme in the Nervous System == |
| - | prevelance of dementias such as Alzheimers Syndrome, led scientists to study the animal enzyme ''AcetylCholinEsterase'' ('''AChE''') as a possible cause. This enzyme rapidly degrades or hydrolizes the neurotransmitter acetylcholine in synapses (junctions between nerve cells) of cholinergic nerve pathways into acetic acid and choline, to turn off the chemical signal for the nerve to fire. Should something happen to deactivate or kill this vital enzyme, nervous paralysis of vital functions occurs, leading to rapid death. Although AChE is apparently not the cause of Alzheimers, it does seem to play a minor role, in that weak inibitory drugs such as Tacrine, E2020 (Aricept) and the natural Chinese natural produce Huperzine appear to delay symptoms. Furthermore, this enzyme is a key target of some very important nerve gasses and related insecticides. Furthermore, it is a pretty fascinating enzyme to study.
| + | Solution of the three-dimensional (3D) structure of [http://en.wikipedia.org/wiki/Pacific_electric_ray ''Torpedo californica''] [[acetylcholinesterase]] (''Tc''AChE) in 1991 opened up new horizons in research on an [http://en.wikipedia.org/wiki/Enzyme enzyme] that had already been the subject of intensive investigation.<ref>PMID:1678899</ref> The unanticipated structure of this extremely rapid enzyme, in which the [http://en.wikipedia.org/wiki/Active_site active site] was found to be buried at the bottom of a <scene name='2ace/Active_site/3'>deep and narrow gorge</scene>, lined by <scene name='2ace/Active_site/4'>14 aromatic residues</scene> <font color='darkmagenta'><b>(colored dark magenta)</b></font>, led to a revision of the views then held concerning [http://en.wikipedia.org/wiki/Substrate_(biochemistry) substrate] traffic, recognition and hydrolysis.<ref>PMID:10545346</ref> To understand how those aromatic residues behave with the enzyme, see [[Flexibility of aromatic residues in acetylcholinesterase]]. Solution of the 3D structure of acetylcholinesterase led to a series of theoretical and experimental studies, which took advantage of recent advances in theoretical techniques for treatment of [http://en.wikipedia.org/wiki/Protein proteins], such as |
| | + | [http://en.wikipedia.org/wiki/Molecular_dynamics molecular dynamics] and [http://en.wikipedia.org/wiki/Electrostatics electrostatics] and to [http://en.wikipedia.org/wiki/Site-directed_mutagenesis site-directed mutagenesis], utilizing suitable expression systems. |
| | | | |
| - | Because of the relative ease in obtaining purified protein in abundance, AceytlCholinesterase was first crystallized by Joel Sussman of the Weizmann Institute, Rehovot Israel, after being extracted from the electric organ of the Pacific Sting Ray, '''Torpedo Californica''', order to determine its detailed
| + | [[Image:Synapse_Schematic.jpg|thumb|Cholinergic Synapse|300px|left]] |
| - | 3-dimensional structure by X-ray crystallography ([[2ace]]). Subsequently its X-ray structure has been determined from over 20 species, ranging from the fruit fly '''Drosophila''' to human.
| + | |
| | + | [http://en.wikipedia.org/wiki/Acetylcholinesterase Acetylcholinesterase] [http://en.wikipedia.org/wiki/Hydrolysis hydrolysizes] the [http://en.wikipedia.org/wiki/Neurotransmitter neurotransmitter] [http://en.wikipedia.org/wiki/Acetylcholine acetylcholine] <scene name='2ace/Cv/2'>(ACh)</scene>, producing <scene name='2ace/Cv/3'>choline and an acetate</scene> group. ACh directly binds <scene name='22/22/Cv/1'>Ser200</scene> (via its [http://en.wikipedia.org/wiki/Nucleophile nucleophilic] Oγ atom) within the <scene name='2ace/Cv/5'>catalytic triad (Ser200, His440, and Glu327)</scene> (ACh/''Tc''AChE structure [[2ace]]). The residues <scene name='2ace/Cv/6'>Trp84 and Phe330</scene> are also important in the [http://en.wikipedia.org/wiki/Ligand ligand] recognition <ref name="Raves">PMID:8989325</ref>. After this binding acetylcholinesterase <scene name='2ace/Cv/7'>hydrolysizes</scene> ACh. <br /> |
| | + | See also [[Acetylcholinesterase with acetylcholine]]. |
| | | | |
| - | <applet load='1ea5' size='200' color='white' frame='true' spin='off' caption='Acetycholinesterase' align='right' />
| + | == Treatment of Alzheimer's disease == |
| - | Acetylcholinesterase is a fairly large protein, consisting of at least 535 amino acid residues in a single peptide chain, that folds into a single protein domain without any apparent symmetry. <scene name='Acetylcholinesterase/1eaf_2ndary/1'>At its core are two large Beta sheets (green), surrounded by a canopy of about 26 alpha helices (red). </scene>
| + | |
| | | | |
| - | The active site region of this enzyme has two sites, a catalytic site and a peripheral site, which helps prebind the substrate and direct it toward the active site. When the 3-D structure was first determined, the big surprise was
| + | [http://en.wikipedia.org/wiki/Alzheimer's_disease Alzheimer's disease] (AD) is a disorder that attacks the [http://en.wikipedia.org/wiki/Central_nervous_system central nervous system] through progressive degeneration of its neurons. AD occurs in around 10% of the elderly and, as yet, there is no known cure. Patients with this disease develop [http://en.wikipedia.org/wiki/Dementia dementia] which becomes more severe as the disease progresses. It was suggested that symptoms of AD are caused by decrease of activity of [http://en.wikipedia.org/wiki/Cholinergic cholinergic] [http://en.wikipedia.org/wiki/Neocortex neocortical] and [http://en.wikipedia.org/wiki/Hippocampus hippocampal] neurons. Treatment of AD by ACh precursors and [http://en.wikipedia.org/wiki/Cholinergic cholinergic] [http://en.wikipedia.org/wiki/Agonist agonists] was ineffective or caused severe side effects. ACh hydrolysis by AChE causes termination of cholinergic neurotransmission. Therefore, compounds which inhibit AChE might significantly increase the levels of ACh depleted in AD. Indeed, it was shown that [http://en.wikipedia.org/wiki/Acetylcholinesterase_inhibitor AChE inhibitors] improve the cognitive abilities of AD patients at early stages of the disease development. The way in which the various cholinesterase inhibitors interact with AChE can be see at:<br /> |
| - | that the active site was deep inside the protein, at the end or base of a | + | *[[Acetylcholinesterase: Treatment of Alzheimer's disease]].<br /> |
| - | <scene name='Acetylcholinesterase/AChE_gorge/1'>long tunnel or gorge </scene>,
| + | *[[Acetylcholinesterase complexed with N-9-(1',2',3',4'-tetrahydroacridinyl)-1,8-diaminooctane]].<br /> |
| - | lined with aromatic residues, with the peripheral site at the top or lip of this gorge. Amazingly, there were no acidic or negatively charged
| + | |
| - | residues anywhere in these 2 sites or along this gorge, as would be expected to
| + | |
| - | help attract and bind the basic, positively charged acetylcholine substrate, although are are some acidic residues nearby. Instead, bulky aromatic residues <scene name='Acetylcholinesterase/1ea5_279_84/2'>Trp 279 and Tyr 121 dominate the peripheral site, and Trp 84 and Phe 330 the active site, together with His 440. </scene> (These numbers are the sequential numbering
| + | |
| - | of the residues, starting from the N-terminus, according to the '''Torpedo Californica''' form of the enzyme.) | + | |
| | | | |
| - | It appears that the principal interaction between the aceylcholine and the enzyme is relatively newly discovered cation-pi interactions between the cationic moiety of the substrate and the many aromatic residues lining the catalytic gorge. Unlike most
| + | == Organophosphorus acid anhydride nerve agents == |
| - | interatomic interactions in chemistry, cation-pi interactions are unusual in that their energy hardly changes as the cationic and
| + | |
| - | aromatic ring centers vary between 4 and 7 Angstroms apart, and for a wide variety of relative orientations of the aromatic rings.
| + | |
| - | This gives the substrate an energetically smooth ride down the gorge with few bumps or
| + | |
| | | | |
| - | Most acetylcholinesterases have a net negative charge and a large patch of negative potential around the entrance to the active site gorge, which may be usefull to attract the positively charged acetycholine substrate to the site. As one travels down the gorge, this potential becomes increasingly more and more negative, reaching a peak at the active site at the base. Because of this potential, the peripherial site is thought to act like a substrate trap, that forces practically molecule of substrate that reaches
| + | [http://en.wikipedia.org/wiki/Organophosphorus Organophosphorus] (OP) [http://en.wikipedia.org/wiki/Acid_anhydride acid anhydride] [http://en.wikipedia.org/wiki/Nerve_agent nerve agents] are potent inhibitors which rapidly phosphonylate AChE and then may undergo an internal dealkylation reaction (called "aging") to produce an OP-enzyme conjugate that cannot be reactivated. |
| - | the peripheral site to travel down the gorge to the active site, that probably contributes greatly to the extremely rapid rate of degrading the substrate. This whole enzyme therefore acts like a brilliantly designed natural vacuum cleaner that clears the | + | As was mentioned above, AChE hydrolysizes the neurotransmitter <scene name='2wfz/Al/2'>ACh</scene>, producing <scene name='2wfz/Al/3'>choline and an acetate</scene> group. <scene name='2wfz/Al/2'>ACh</scene> directly binds catalytic <scene name='2wfz/Al/4'>Ser200</scene> (via its nucleophilic Oγ atom). <scene name='2wfz/Al/5'>Soman</scene>, [http://en.wikipedia.org/wiki/Soman O-(1,2,2-trimethylpropyl) methylphosphonofluoridate] (<font color='violet'><b>fluorine atom is colored violet</b></font> and <font color='darkmagenta'><b>phosphorus atom is colored darkmagenta</b></font>), is one of the most toxic OPs. Soman inhibits AChE by <scene name='2wfz/Al/6'>covalent binding</scene> to catalytic Ser200, <scene name='2wfz/Al/7'>mimicking ACh</scene>. This process <scene name='2wfz/Al/8'>(phosphonylation)</scene> implicates nucleophilic attack of the Ser200 nucleophilic Oγ atom on the phosphorus atom of soman, with concomitant departure of its fluoride atom. After that AChE catalyzes the <scene name='2wfz/Al/9'>dealkylation</scene> ("aging") of the soman or other OP. This causes irreversible inhibition of AChE, "aged" soman/AChE conjugate can not be reactivated. However, before “aging”, at the step of <scene name='2wfz/Al/8'>phosphonylation</scene>, AChE can be <scene name='2wfz/Al/11'>reactivated</scene> by nucleophiles, such as pralidoxime (2-PAM), resulting in <scene name='2wfz/Al/12'>cleavage</scene> of the phosphonyl adduct from Ser200 Oγ. |
| - | neurotransmitter out of the synapse extremely quickly. Yet to be solved, however, is how the products clear the active site rapidly,
| + | At the <scene name='2wfz/Ali/3'>active site of the nonaged soman/TcAChE conjugate</scene> ([[2wfz]]) the catalytic His440 forms hydrogen bonds with Ser200 Oγ and Glu327 Oε1 via its Nε2 and Nδ1 nitrogens, respectively. The O2 atom of soman is within hydrogen bonding distance of His440 Nε2. Soman O1 mimicks carbonyl oxygen of ACh. A water molecule 1001 interacting with soman O2 is represented as a <font color='red'><b>red ball</b></font>. The active site residues of the nonaged soman/TcAChE are colored <span style="color:yellow;background-color:black;font-weight:bold;">yellow</span>. The O2 atom of the <scene name='2wfz/Ali/4'>dealkylated (aged) soman</scene> ([[2wg0]]) forms a salt bridge with His440 Nε2. The active site residues of the aged soman/TcAChE are colored <span style="color:pink;background-color:black;font-weight:bold;">pink</span>. <scene name='2wfz/Ali/5'>Alignment</scene> of the structures of the nonaged ([[2wfz]]) and aged ([[2wg0]]) conjugates reveals a small, but important, change within the active site - the imidazole ring of His440 is tilted back to a native-like conformation after dealkylation. The water molecule 1001, which interacts with soman O2 in the nonaged crystal structure, is not within hydrogen bonding distance of O2 in the aged crystal structure. 2-PAM binds poorly to the nonaged phosphonylated enzyme (its electron density was not found) and binds in an <scene name='2wfz/Ali/7'>unfavorable and nonfunctional conformation</scene> after soman aging to ''Tc''AChE ([[2wg1]]) <ref name="Sanson">PMID:19642642</ref>. |
| - | whether back through the gorge, or out a back door on the other side of the protein that quickly opens each catalytic cycle (Try 84
| + | |
| - | is actually near the surface of the 'underside' of the protein.)
| + | |
| | | | |
| - | ==Selected structures== | + | ==Additional resources== |
| - | * [[2ace]] This is the original solved structure for '''Torpedo Californica'''
| + | see: [[Acetylcholinesterase_Additional_Resources]] |
| - | * [[1ea5]] This is one of the highest quality representative X-ray structures in the PDB.
| + | |
| | | | |
| - | ==PDBs containing acetylcholinesterase== | + | ==Movies== |
| | + | see: [[Acetylcholinesterase_Movies]] |
| | + | |
| | + | ==Acetylcholinesterase 3D structures== |
| | + | |
| | + | [[Acetylcholinesterase 3D structures]] |
| | + | |
| | + | ==References== |
| | + | <references/> |
| | + | |
| | + | </StructureSection> |
| | | | |
| | [[Category: catalytic triad]] | | [[Category: catalytic triad]] |
| | + | [[Category: cholinesterase]] |
| | [[Category: acetylcholine]] | | [[Category: acetylcholine]] |
| | [[Category: cation-pi]] | | [[Category: cation-pi]] |
| | [[Category: Alzheimers]] | | [[Category: Alzheimers]] |
| | [[Category: nerve gasses]] | | [[Category: nerve gasses]] |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | [[Category:Topic Page]] |
|
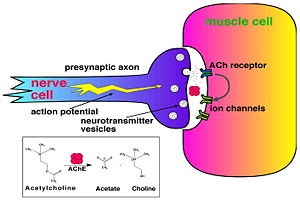
Acetylcholinesterase (AChE) is key enzyme in the nervous system of animals. By rapid hydrolysis of the neurotransmitter, acetylcholine (ACh), AChE terminates neurotransmission at cholinergic synapses. It is a very fast enzyme, especially for a serine hydrolase, functioning at a rate approaching that of a diffusion-controlled reaction. AChE inhibitors are among the key drugs approved by the FDA for management of Alzheimer's disease (AD). The powerful toxicity of organophosphorus (OP) poisons is attributed primarily to their potent AChE inhibitors.
See also Acetylcholinesterase (Hebrew)
Key Enzyme in the Nervous System
Solution of the three-dimensional (3D) structure of Torpedo californica acetylcholinesterase (TcAChE) in 1991 opened up new horizons in research on an enzyme that had already been the subject of intensive investigation.[1] The unanticipated structure of this extremely rapid enzyme, in which the active site was found to be buried at the bottom of a , lined by (colored dark magenta), led to a revision of the views then held concerning substrate traffic, recognition and hydrolysis.[2] To understand how those aromatic residues behave with the enzyme, see Flexibility of aromatic residues in acetylcholinesterase. Solution of the 3D structure of acetylcholinesterase led to a series of theoretical and experimental studies, which took advantage of recent advances in theoretical techniques for treatment of proteins, such as
molecular dynamics and electrostatics and to site-directed mutagenesis, utilizing suitable expression systems.
Acetylcholinesterase hydrolysizes the neurotransmitter acetylcholine , producing group. ACh directly binds (via its nucleophilic Oγ atom) within the (ACh/TcAChE structure 2ace). The residues are also important in the ligand recognition [3]. After this binding acetylcholinesterase ACh.
See also Acetylcholinesterase with acetylcholine.
Treatment of Alzheimer's disease
Alzheimer's disease (AD) is a disorder that attacks the central nervous system through progressive degeneration of its neurons. AD occurs in around 10% of the elderly and, as yet, there is no known cure. Patients with this disease develop dementia which becomes more severe as the disease progresses. It was suggested that symptoms of AD are caused by decrease of activity of cholinergic neocortical and hippocampal neurons. Treatment of AD by ACh precursors and cholinergic agonists was ineffective or caused severe side effects. ACh hydrolysis by AChE causes termination of cholinergic neurotransmission. Therefore, compounds which inhibit AChE might significantly increase the levels of ACh depleted in AD. Indeed, it was shown that AChE inhibitors improve the cognitive abilities of AD patients at early stages of the disease development. The way in which the various cholinesterase inhibitors interact with AChE can be see at:
Organophosphorus acid anhydride nerve agents
Organophosphorus (OP) acid anhydride nerve agents are potent inhibitors which rapidly phosphonylate AChE and then may undergo an internal dealkylation reaction (called "aging") to produce an OP-enzyme conjugate that cannot be reactivated.
As was mentioned above, AChE hydrolysizes the neurotransmitter , producing group. directly binds catalytic (via its nucleophilic Oγ atom). , O-(1,2,2-trimethylpropyl) methylphosphonofluoridate (fluorine atom is colored violet and phosphorus atom is colored darkmagenta), is one of the most toxic OPs. Soman inhibits AChE by to catalytic Ser200, . This process implicates nucleophilic attack of the Ser200 nucleophilic Oγ atom on the phosphorus atom of soman, with concomitant departure of its fluoride atom. After that AChE catalyzes the ("aging") of the soman or other OP. This causes irreversible inhibition of AChE, "aged" soman/AChE conjugate can not be reactivated. However, before “aging”, at the step of , AChE can be by nucleophiles, such as pralidoxime (2-PAM), resulting in of the phosphonyl adduct from Ser200 Oγ.
At the (2wfz) the catalytic His440 forms hydrogen bonds with Ser200 Oγ and Glu327 Oε1 via its Nε2 and Nδ1 nitrogens, respectively. The O2 atom of soman is within hydrogen bonding distance of His440 Nε2. Soman O1 mimicks carbonyl oxygen of ACh. A water molecule 1001 interacting with soman O2 is represented as a red ball. The active site residues of the nonaged soman/TcAChE are colored yellow. The O2 atom of the (2wg0) forms a salt bridge with His440 Nε2. The active site residues of the aged soman/TcAChE are colored pink. of the structures of the nonaged (2wfz) and aged (2wg0) conjugates reveals a small, but important, change within the active site - the imidazole ring of His440 is tilted back to a native-like conformation after dealkylation. The water molecule 1001, which interacts with soman O2 in the nonaged crystal structure, is not within hydrogen bonding distance of O2 in the aged crystal structure. 2-PAM binds poorly to the nonaged phosphonylated enzyme (its electron density was not found) and binds in an after soman aging to TcAChE (2wg1) [4].
Additional resources
see: Acetylcholinesterase_Additional_Resources
Movies
see: Acetylcholinesterase_Movies
Acetylcholinesterase 3D structures
Acetylcholinesterase 3D structures
References
- ↑ Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872-9. PMID:1678899
- ↑ Botti SA, Felder CE, Lifson S, Sussman JL, Silman I. A modular treatment of molecular traffic through the active site of cholinesterase. Biophys J. 1999 Nov;77(5):2430-50. PMID:10545346
- ↑ Raves ML, Harel M, Pang YP, Silman I, Kozikowski AP, Sussman JL. Structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A. Nat Struct Biol. 1997 Jan;4(1):57-63. PMID:8989325
- ↑ Sanson B, Nachon F, Colletier JP, Froment MT, Toker L, Greenblatt HM, Sussman JL, Ashani Y, Masson P, Silman I, Weik M. Crystallographic Snapshots of Nonaged and Aged Conjugates of Soman with Acetylcholinesterase, and of a Ternary Complex of the Aged Conjugate with Pralidoxime (dagger). J Med Chem. 2009 Jul 30. PMID:19642642 doi:10.1021/jm900433t
|