We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
1diz
From Proteopedia
(Difference between revisions)
(New page: 200px<br /><applet load="1diz" size="450" color="white" frame="true" align="right" spinBox="true" caption="1diz, resolution 2.50Å" /> '''CRYSTAL STRUCTURE OF...) |
|||
| (18 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | [[Image:1diz.gif|left|200px]]<br /><applet load="1diz" size="450" color="white" frame="true" align="right" spinBox="true" | ||
| - | caption="1diz, resolution 2.50Å" /> | ||
| - | '''CRYSTAL STRUCTURE OF E. COLI 3-METHYLADENINE DNA GLYCOSYLASE (ALKA) COMPLEXED WITH DNA'''<br /> | ||
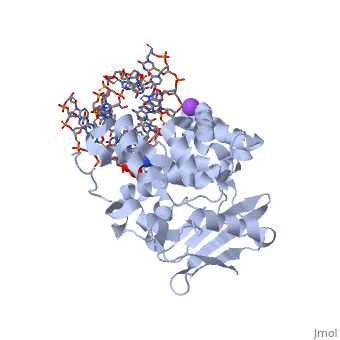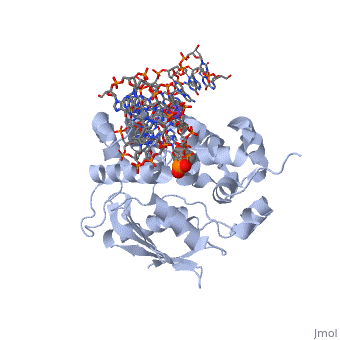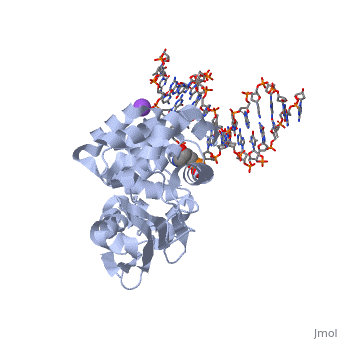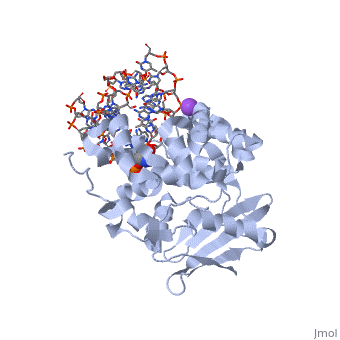
| - | == | + | ==CRYSTAL STRUCTURE OF E. COLI 3-METHYLADENINE DNA GLYCOSYLASE (ALKA) COMPLEXED WITH DNA== |
| - | + | <StructureSection load='1diz' size='340' side='right'caption='[[1diz]], [[Resolution|resolution]] 2.50Å' scene=''> | |
| + | == Structural highlights == | ||
| + | <table><tr><td colspan='2'>[[1diz]] is a 6 chain structure with sequence from [https://en.wikipedia.org/wiki/Escherichia_coli Escherichia coli]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1DIZ OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1DIZ FirstGlance]. <br> | ||
| + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.5Å</td></tr> | ||
| + | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=NA:SODIUM+ION'>NA</scene>, <scene name='pdbligand=NRI:PHOSPHORIC+ACID+MONO-(4-HYDROXY-PYRROLIDIN-3-YLMETHYL)+ESTER'>NRI</scene></td></tr> | ||
| + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1diz FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1diz OCA], [https://pdbe.org/1diz PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1diz RCSB], [https://www.ebi.ac.uk/pdbsum/1diz PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1diz ProSAT]</span></td></tr> | ||
| + | </table> | ||
| + | == Function == | ||
| + | [https://www.uniprot.org/uniprot/3MG2_ECOLI 3MG2_ECOLI] Hydrolysis of the deoxyribose N-glycosidic bond to excise 3-methyladenine, 3-methylguanine, 7-methylguanine, O2-methylthymine, and O2-methylcytosine from the damaged DNA polymer formed by alkylation lesions. | ||
| + | == Evolutionary Conservation == | ||
| + | [[Image:Consurf_key_small.gif|200px|right]] | ||
| + | Check<jmol> | ||
| + | <jmolCheckbox> | ||
| + | <scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/di/1diz_consurf.spt"</scriptWhenChecked> | ||
| + | <scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | ||
| + | <text>to colour the structure by Evolutionary Conservation</text> | ||
| + | </jmolCheckbox> | ||
| + | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1diz ConSurf]. | ||
| + | <div style="clear:both"></div> | ||
| - | == | + | ==See Also== |
| - | + | *[[DNA glycosylase 3D structures|DNA glycosylase 3D structures]] | |
| - | + | __TOC__ | |
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
[[Category: Escherichia coli]] | [[Category: Escherichia coli]] | ||
| - | [[Category: | + | [[Category: Large Structures]] |
| - | [[Category: Ellenberger | + | [[Category: Ellenberger TE]] |
| - | [[Category: Hollis | + | [[Category: Hollis T]] |
| - | [[Category: Ichikawa | + | [[Category: Ichikawa Y]] |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Current revision
CRYSTAL STRUCTURE OF E. COLI 3-METHYLADENINE DNA GLYCOSYLASE (ALKA) COMPLEXED WITH DNA
| |||||||||||