|
|
| (33 intermediate revisions not shown.) |
| Line 1: |
Line 1: |
| | + | <StructureSection load="" size="400" color="white" caption="Mouse antizyme inhibitor 1 dimer [[3btn]]" scene='Antizyme_Inhibitor/Azi/1' spinBox="true" > |
| | [[Image:AziFig.PNG|left|300px]] | | [[Image:AziFig.PNG|left|300px]] |
| - | '''Crystal structure of the Antizyme inhibitor'''
| + | ==Antizyme Inhibitor== |
| | + | {{ABSTRACT_PUBMED_18369191}} |
| | {{Clear}} | | {{Clear}} |
| - | {{Structure
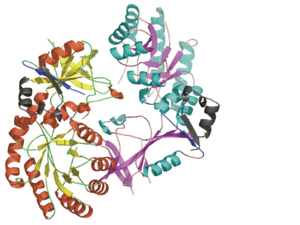
| + | Each <scene name='Antizyme_Inhibitor/Monomer/5'>monomer</scene> consists of two domains: a <scene name='Antizyme_Inhibitor/Monomer/6'>TIM-like</scene> α/β-barrel [http://en.wikipedia.org/wiki/TIM_barrel] domain (residues 45–280) and a modified <scene name='Antizyme_Inhibitor/Monomer/7'>Greek key</scene> [http://en.wikipedia.org/wiki/Greek_key] β-sheet domain (residues 8–44 and 281–435). <font color='red'><b>Helices</b></font> [http://en.wikipedia.org/wiki/Alpha_helix] are colored in <font color='red'><b>red</b></font> and <font color='black'><b>β sheets</b></font> [http://en.wikipedia.org/wiki/Beta_sheet] in <font color='black'><b>yellow</b></font>. |
| - | |PDB=3BTN.pdb|SIZE=350|SCENE=Antizyme_Inhibitor/Azi/1|CAPTION=AzI, unpublished structure
| + | |
| - | |SITE=
| + | |
| - | |LIGAND=
| + | |
| - | |ACTIVITY=
| + | |
| - | |GENE=
| + | |
| - | |DOMAIN=
| + | |
| - | |RELATEDENTRY=
| + | |
| - | |RESOURCES=
| + | |
| - | }}
| + | |
| - | <!-- | + | |
| - | <applet load="3BTN.pdb" size="250" color="white" frame="true" align="right" spinBox="true" | + | |
| - | caption="AzI, unpublished structure" />
| + | |
| - | -->
| + | |
| - | '''Preview.'''
| + | |
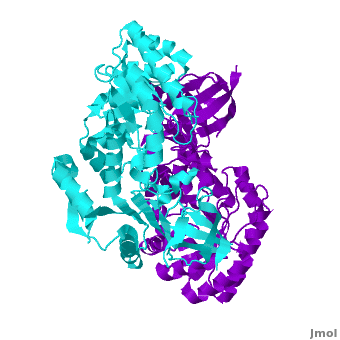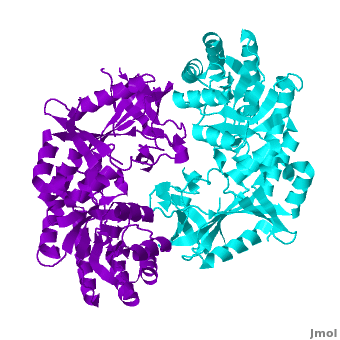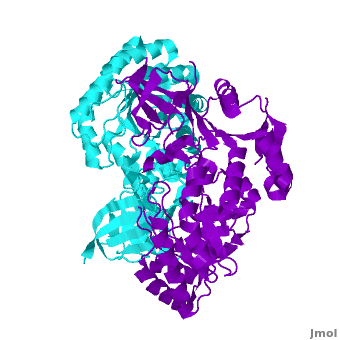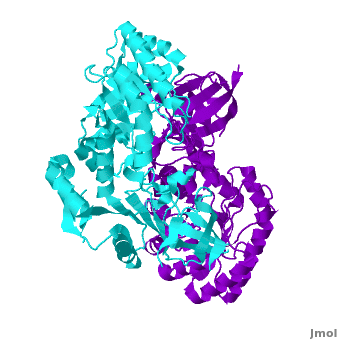
| - | Antizyme inhibitor (AzI) regulates cellular polyamine homeostasis by binding to the polyamine-induced protein, Antizyme (Az), with greater affinity than ODC. AzI is highly homologous to ornithine decarboxylase (ODC), but is not enzymatically active. In order to understand these specific characteristics of AzI and its differences from ODC, we determined the 3D structure of mouse AzI to 2.05Å resolution. Both AzI and ODC crystallize as a
| + | |
| - | <scene name='3btn/Azi/1'>dimer</scene> (one monomer in cyan and the other in blue violet). | + | |
| - | However, fewer interactions at the dimer interface, a smaller buried surface area, and lack of symmetry of the interactions between residues from the two monomers in the AzI structure suggest that this dimeric structure is non-physiological. In addition, the absence of residues and interactions required for PLP binding suggest that AzI does not bind PLP. A comparison to the PLP binding site of ODC revealed that AzI lacks the residues participating in PLP binding. Biochemical studies confirmed the lack of PLP binding and revealed that AzI exists as a monomer in solution while ODC is dimeric. Our findings that AzI exists as a monomer and its inability to bind PLP provide two independent explanations for its lack of enzymatic activity, and suggest the basis for its enhanced affinity towards Az.
| + | |
| | {{Clear}} | | {{Clear}} |
| - | | + | A sequence alignment and structural comparison of mouse AzI crystallographic dimer to mouse, human, and [http://en.wikipedia.org/wiki/Trypanosome trypanosome] [http://en.wikipedia.org/wiki/Ornithine_decarboxylase ODC] (mODC, hODC, and tODC, respectively) show high sequence identity (~50%) and structural similarity between AzI and ODC monomers (RMSD values of 1.85 Å, 1.6 Å, and 1.5 Å, respectively). The <scene name='Antizyme_Inhibitor/Azi_odc/10'>structural comparison</scene> of mouse AzI crystallographic dimer (mAzI, <font color='cyan'><b>cyan</b></font> and <font color='blueviolet'><b>blueviolet</b></font>) to mODC (PDB code [[7odc]], (<font color='red'><b>red</b></font> and <font color='lime'><b>lime</b></font>) is shown. Superposition of the <scene name='Antizyme_Inhibitor/Azi_odc/11'>interface</scene> of mAzI and mODC showing the inter-subunit variable loops (AzI residues 355–362 and 387–401). <font color='black'><b>AzI loops</b></font> are in <font color='black'><b>black</b></font>, and <font color='black'><b>ODC loops</b></font> are in <font color='black'><b>yellow</b></font>. |
| - | <applet load="3BTN.pdb" size="300" color="white" frame="true" align="left" scene='Antizyme_Inhibitor/Azi/1' spinBox="true" /> | + | The two AzI monomers (<font color='cyan'><b>cyan</b></font>, <font color='blueviolet'><b>blueviolet</b></font>) have only <scene name='Antizyme_Inhibitor/Azi_odc1/1'>43 contacting residues</scene> (< 3.5 Å apart), while there are more contacts between the two monomers of hODC, <scene name='Antizyme_Inhibitor/Azi_odc1/2'>mODC</scene> (<font color='red'><b>red</b></font>, <font color='lime'><b>lime</b></font>), and tODC (74, 83 and 69, respectively). Moreover, the surface area buried by the two mODC monomers is significantly larger than the one buried by the AzI monomers. These features explain a very weak crystallographic AzI dimer. |
| - | Each <scene name='Antizyme_Inhibitor/Monomer/2'>monomer</scene> consists of two domains: a <scene name='Antizyme_Inhibitor/Monomer/3'>TIM-like</scene> α/β-barrel [http://en.wikipedia.org/wiki/TIM_barrel] domain (residues 45–280) and a modified <scene name='Antizyme_Inhibitor/Monomer/4'>Greek key</scene> [http://en.wikipedia.org/wiki/Greek_key] β-sheet domain (residues 8–44 and 281–435). Helices are colored in red and β strands in yellow.
| + | The zipper, formed by conserved [http://en.wikipedia.org/wiki/Hydrophobe hydrophobic] residues in mODC, stabilizes its dimeric structure. These residues involve F397(B), Y323(B), Y331(A), Y331(B), Y323(A), and F397(A) (the names of the chains are in brackets). The residue Y331 in the <scene name='Antizyme_Inhibitor/Azi_odc1/3'>ODC zipper</scene> is substituted by S329 in AzI and interferes with the formation of a similar zipper in AzI. Hence, in <scene name='Antizyme_Inhibitor/Azi_odc1/4'>mAzI</scene> this hydrophobic zipper is absent. Many residues, participating in the ODC interdimer interface interactions, are conserved among the ODCs from variuos organisms, but in AzI these residues are not conserved. Furthermore, the AzI conserved residues do not participate in interdimer interactions. For example, mODC possesses <scene name='Antizyme_Inhibitor/Azi_odc1/5'>two salt bridges</scene> (K169–D364 and D134–K294) stabilizing the ODC [http://en.wikipedia.org/wiki/Dimer homodimer]. In AzI, these 4 corresponding residues (<scene name='Antizyme_Inhibitor/Azi_odc1/6'>K169-D362 and D134-K291</scene>, respectively) are also present, but are too far apart to form a salt bridge. The two AzI monomers are positioned farther apart, in comparison ot ODC monomers, preventing the formation of interdimer interactions. |
| | {{Clear}} | | {{Clear}} |
| | | | |
| | + | <scene name='Antizyme_Inhibitor/Binding_site/6'>Overlap</scene> of the AzI and mODC structures suggests that AzI does not bind [http://en.wikipedia.org/wiki/Pyridoxal_phosphate PLP]. <font color='black'><b>PLP</b></font> is in <font color='black'><b>yellow</b></font>, <font color='lime'><b>ODC residues D88, R154, R277, and Y389 are in lime</b></font>, and the corresponding <font color='magenta'><b>AzI residues A88, H154, S274, and D387 are in magenta</b></font>. Many of the residues participating in PLP-ODC binding are not conserved in AzI. These include D88A, R154H, R277S, D332E, and Y389D (ODC residue numbers follow the sequence of AzI). Notably, the absence of even one of these interactions, (''e.g.'' ODC R277A mutant) results in a 100-fold decrease in PLP binding, a 50% drop in K<sub>cat</sub>, and a 7-fold decrease in K<sub>M</sub>. |
| | | | |
| - | <applet load="AzIODCali.pdb" size="500" color="white" frame="true" align="right" scene='Antizyme_Inhibitor/Azi_odc/1' spinBox="true" />
| + | ==3D structures of Antizyme Inhibitor== |
| | + | [[Antizyme inhibitor 3D structures]] |
| | | | |
| | + | </StructureSection> |
| | | | |
| - | '''Comparison of the AzI structure to that of ODC.'''
| + | ==Additional Resources== |
| - | A primary sequence alignment and <scene name='Antizyme_Inhibitor/Azi_odc/3'>structural comparison</scene> of mouse AzI crystallographic dimer (mAzI, cyan and blue violet) to mouse, human, and trypanosome ODC (mODC (PDB code [[7odc]], red and lime), hODC, and tODC, respectively) reveal high sequence identity (~50%) and structural similarity between AzI and ODC monomers (RMSD values of 1.85 Å, 1.6 Å, and 1.5 Å, respectively). Superposition of the <scene name='Antizyme_Inhibitor/Azi_odc/2'>interface</scene> of mAzI and mODC showing the variable loops between monomers A and B and between monomers A and B (AzI residues 355–362 and 387–401). AzI loops are in black, and ODC loops are in yellow.
| + | For additional information, see: [[Cancer]] |
| - | {{Clear}}
| + | <br /> |
| - | {{Clear}}
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | '''Structure of AzI suggests its existence as a monomer in solution.'''
| + | |
| - | AzI crystallizes as a dimer such that the two monomers adopt a head-to-tail orientation as observed in the ODC dimer. The two monomers of AzI exhibit only <scene name='Antizyme_Inhibitor/Azi_odc/4'>43 contacts</scene> (up 3.5 Å), while significantly more contacts are observed between the two monomers of hODC, mODC, and tODC (83, <scene name='Antizyme_Inhibitor/Azi_odc/5'>74</scene>, and 69, respectively). Furthermore, the surface area buried by the two AzI monomers is smaller than that buried by the mODC monomers (1492 Å<sup>2</sup> per monomer compared to 2284 Å<sup>2</sup>, which are 8% and 13% of the solvent accessible area, respectively). These properties support a very loose crystallographic dimer.
| + | |
| - | Conserved hydrophobic residues in ODC form a zipper that stabilizes its homodimeric structure. These residues include F397(B), Y323(B), Y331(A), Y331(B), Y323(A), and F397(A) (A and B correspond to the two monomers, respectively). Y331, an important residue in the <scene name='Antizyme_Inhibitor/Azi_odc/6'>ODC zipper</scene>, is changed to S329 in AzI and interferes with the formation of a similar zipper in AzI. Thus, <scene name='Antizyme_Inhibitor/Azi_odc/7'>mAzI</scene> showing the absence of the hydrophobic zipper. Many of the interactions in the ODC interface involve residues that are conserved among the ODCs from various species but are different in AzI. Moreover, even those that are conserved in AzI do not participate in interdimer interactions. These include the <scene name='Antizyme_Inhibitor/Azi_odc/8'>two salt bridges</scene>, K169–D364 and D134–K294, which stabilize the ODC homodimer. In AzI, all these 4 residues (<scene name='Antizyme_Inhibitor/Azi_odc/9'>K169-D362 and D134-K291</scene>, respectively) are conserved, yet these two salt bridges are not formed. A careful inspection of the AzI dimer interface demonstrates that the two monomers are further apart compared to those of ODC, preventing the formation of these interactions.
| + | |
| - | {{Clear}}
| + | |
| - | | + | |
| - | <applet load="AzIODCplp.pdb" size="500" color="white" frame="true" align="right" scene='Antizyme_Inhibitor/Binding_site/5' spinBox="true" />
| + | |
| - | | + | |
| - | '''AzI does not bind PLP.'''
| + | |
| - | PLP-dependent enzymes have conserved active-site residues and secondary-structural features, implying that they have similar PLP binding sites. A comparative analysis of the structures of AzI versus those of ODC suggests that AzI does not bind PLP. Many of the residues involved in mediating PLP binding in ODC are not conserved in AzI. These include D88A, R154H, R277S, D332E, and Y389D (ODC residue numbering followed by the amino acid in AzI). Thus, the environment formed by AzI is different than that of ODC, possibly preventing important interactions between AzI and PLP. It is important to note in this respect that the loss of even one of these interactions, as exemplified in the ODC R277A mutant, results in a 100-fold decrease in PLP binding as well as a 50% drop in K<sub>cat</sub> and a sevenfold decrease in K<sub>M</sub>. Comparison of <scene name='Antizyme_Inhibitor/Binding_site/4'>PLP-binding site</scene> in ODC to the corresponding residues in AzI. PLP is in yellow, ODC residues D88, R154, R277, and Y389 are in green, and corresponding AzI residues A88, H154, S274, and D387 are in magenta.
| + | |
| - | {{Clear}}
| + | |
| | | | |
| - | ==Primary Reference== | + | ==Reference== |
| | Shira Albeck, Orly Dym, Tamar Unger, Zohar Snapir, Zippy Bercovich and Chaim Kahana. Crystallographic and biochemical studies revealing the structural basis for antizyme inhibitor function. [http://www.ncbi.nlm.nih.gov/pubmed/18369191?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum Protein Sci. 2008 May; 17(5): 793-802. Epub 2008 Mar 27.] | | Shira Albeck, Orly Dym, Tamar Unger, Zohar Snapir, Zippy Bercovich and Chaim Kahana. Crystallographic and biochemical studies revealing the structural basis for antizyme inhibitor function. [http://www.ncbi.nlm.nih.gov/pubmed/18369191?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum Protein Sci. 2008 May; 17(5): 793-802. Epub 2008 Mar 27.] |
| | [[Category: Albeck, S.]] | | [[Category: Albeck, S.]] |
| Line 54: |
Line 28: |
| | [[Category: Unger, T.]] | | [[Category: Unger, T.]] |
| | [[Category: ISPC, Israel Structural Proteomics Center.]] | | [[Category: ISPC, Israel Structural Proteomics Center.]] |
| - | [[Category: Ispc]] | + | [[Category: ISPC]] |
| | [[Category: Israel structural proteomics center]] | | [[Category: Israel structural proteomics center]] |
| | [[Category: Structural genomic]] | | [[Category: Structural genomic]] |
| | + | [[Category:Topic Page]] |
|
Antizyme Inhibitor
Publication Abstract from PubMed
Antizyme inhibitor (AzI) regulates cellular polyamine homeostasis by binding to the polyamine-induced protein, Antizyme (Az), with greater affinity than ornithine decarboxylase (ODC). AzI is highly homologous to ODC but is not enzymatically active. In order to understand these specific characteristics of AzI and its differences from ODC, we determined the 3D structure of mouse AzI to 2.05 A resolution. Both AzI and ODC crystallize as a dimer. However, fewer interactions at the dimer interface, a smaller buried surface area, and lack of symmetry of the interactions between residues from the two monomers in the AzI structure suggest that this dimeric structure is nonphysiological. In addition, the absence of residues and interactions required for pyridoxal 5'-phosphate (PLP) binding suggests that AzI does not bind PLP. Biochemical studies confirmed the lack of PLP binding and revealed that AzI exists as a monomer in solution while ODC is dimeric. Our findings that AzI exists as a monomer and is unable to bind PLP provide two independent explanations for its lack of enzymatic activity and suggest the basis for its enhanced affinity toward Az.
Crystallographic and biochemical studies revealing the structural basis for antizyme inhibitor function., Albeck S, Dym O, Unger T, Snapir Z, Bercovich Z, Kahana C, Protein Sci. 2008 May;17(5):793-802. Epub 2008 Mar 27. PMID:18369191
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
Each consists of two domains: a α/β-barrel [1] domain (residues 45–280) and a modified [2] β-sheet domain (residues 8–44 and 281–435). Helices [3] are colored in red and β sheets [4] in yellow.
A sequence alignment and structural comparison of mouse AzI crystallographic dimer to mouse, human, and trypanosome ODC (mODC, hODC, and tODC, respectively) show high sequence identity (~50%) and structural similarity between AzI and ODC monomers (RMSD values of 1.85 Å, 1.6 Å, and 1.5 Å, respectively). The of mouse AzI crystallographic dimer (mAzI, cyan and blueviolet) to mODC (PDB code 7odc, (red and lime) is shown. Superposition of the of mAzI and mODC showing the inter-subunit variable loops (AzI residues 355–362 and 387–401). AzI loops are in black, and ODC loops are in yellow.
The two AzI monomers (cyan, blueviolet) have only (< 3.5 Å apart), while there are more contacts between the two monomers of hODC, (red, lime), and tODC (74, 83 and 69, respectively). Moreover, the surface area buried by the two mODC monomers is significantly larger than the one buried by the AzI monomers. These features explain a very weak crystallographic AzI dimer.
The zipper, formed by conserved hydrophobic residues in mODC, stabilizes its dimeric structure. These residues involve F397(B), Y323(B), Y331(A), Y331(B), Y323(A), and F397(A) (the names of the chains are in brackets). The residue Y331 in the is substituted by S329 in AzI and interferes with the formation of a similar zipper in AzI. Hence, in this hydrophobic zipper is absent. Many residues, participating in the ODC interdimer interface interactions, are conserved among the ODCs from variuos organisms, but in AzI these residues are not conserved. Furthermore, the AzI conserved residues do not participate in interdimer interactions. For example, mODC possesses (K169–D364 and D134–K294) stabilizing the ODC homodimer. In AzI, these 4 corresponding residues (, respectively) are also present, but are too far apart to form a salt bridge. The two AzI monomers are positioned farther apart, in comparison ot ODC monomers, preventing the formation of interdimer interactions.
of the AzI and mODC structures suggests that AzI does not bind PLP. PLP is in yellow, ODC residues D88, R154, R277, and Y389 are in lime, and the corresponding AzI residues A88, H154, S274, and D387 are in magenta. Many of the residues participating in PLP-ODC binding are not conserved in AzI. These include D88A, R154H, R277S, D332E, and Y389D (ODC residue numbers follow the sequence of AzI). Notably, the absence of even one of these interactions, (e.g. ODC R277A mutant) results in a 100-fold decrease in PLP binding, a 50% drop in Kcat, and a 7-fold decrease in KM.
3D structures of Antizyme Inhibitor
Antizyme inhibitor 3D structures
|
Shira Albeck, Orly Dym, Tamar Unger, Zohar Snapir, Zippy Bercovich and Chaim Kahana. Crystallographic and biochemical studies revealing the structural basis for antizyme inhibitor function. Protein Sci. 2008 May; 17(5): 793-802. Epub 2008 Mar 27.