|
|
| (24 intermediate revisions not shown.) |
| Line 1: |
Line 1: |
| | + | <StructureSection load="" size="400" color="white" caption="Mouse antizyme inhibitor 1 dimer [[3btn]]" scene='Antizyme_Inhibitor/Azi/1' spinBox="true" > |
| | [[Image:AziFig.PNG|left|300px]] | | [[Image:AziFig.PNG|left|300px]] |
| - | '''Crystal structure of the Antizyme inhibitor'''
| + | ==Antizyme Inhibitor== |
| - | {{Clear}}
| + | |
| - | {{Structure
| + | |
| - | |PDB=3BTN.pdb|SIZE=350|SCENE=Antizyme_Inhibitor/Azi/1|CAPTION=AzI, unpublished structure
| + | |
| - | |SITE=
| + | |
| - | |LIGAND=
| + | |
| - | |ACTIVITY=
| + | |
| - | |GENE=
| + | |
| - | |DOMAIN=
| + | |
| - | |RELATEDENTRY=
| + | |
| - | |RESOURCES=
| + | |
| - | }}
| + | |
| - | <!--
| + | |
| - | <applet load="3BTN.pdb" size="250" color="white" frame="true" align="right" spinBox="true"
| + | |
| - | caption="AzI, unpublished structure" />
| + | |
| - | -->
| + | |
| | {{ABSTRACT_PUBMED_18369191}} | | {{ABSTRACT_PUBMED_18369191}} |
| - | | |
| - | | |
| - | | |
| | {{Clear}} | | {{Clear}} |
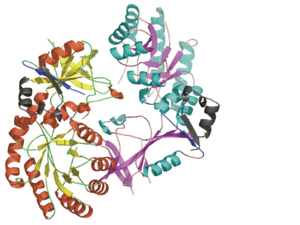
| - | | + | Each <scene name='Antizyme_Inhibitor/Monomer/5'>monomer</scene> consists of two domains: a <scene name='Antizyme_Inhibitor/Monomer/6'>TIM-like</scene> α/β-barrel [http://en.wikipedia.org/wiki/TIM_barrel] domain (residues 45–280) and a modified <scene name='Antizyme_Inhibitor/Monomer/7'>Greek key</scene> [http://en.wikipedia.org/wiki/Greek_key] β-sheet domain (residues 8–44 and 281–435). <font color='red'><b>Helices</b></font> [http://en.wikipedia.org/wiki/Alpha_helix] are colored in <font color='red'><b>red</b></font> and <font color='black'><b>β sheets</b></font> [http://en.wikipedia.org/wiki/Beta_sheet] in <font color='black'><b>yellow</b></font>. |
| - | <applet load="3BTN.pdb" size="300" color="white" frame="true" align="left" scene='Antizyme_Inhibitor/Azi/1' spinBox="true" />
| + | {{Clear}} |
| - | Each <scene name='Antizyme_Inhibitor/Monomer/5'>monomer</scene> consists of two domains: a <scene name='Antizyme_Inhibitor/Monomer/6'>TIM-like</scene> α/β-barrel [http://en.wikipedia.org/wiki/TIM_barrel] domain (residues 45–280) and a modified <scene name='Antizyme_Inhibitor/Monomer/7'>Greek key</scene> [http://en.wikipedia.org/wiki/Greek_key] β-sheet domain (residues 8–44 and 281–435). <font color='red'><b>Helices</b></font> are colored in <font color='red'><b>red</b></font> and <font color='black'><b>β strands</b></font> in <font color='black'><b>yellow</b></font>. | + | A sequence alignment and structural comparison of mouse AzI crystallographic dimer to mouse, human, and [http://en.wikipedia.org/wiki/Trypanosome trypanosome] [http://en.wikipedia.org/wiki/Ornithine_decarboxylase ODC] (mODC, hODC, and tODC, respectively) show high sequence identity (~50%) and structural similarity between AzI and ODC monomers (RMSD values of 1.85 Å, 1.6 Å, and 1.5 Å, respectively). The <scene name='Antizyme_Inhibitor/Azi_odc/10'>structural comparison</scene> of mouse AzI crystallographic dimer (mAzI, <font color='cyan'><b>cyan</b></font> and <font color='blueviolet'><b>blueviolet</b></font>) to mODC (PDB code [[7odc]], (<font color='red'><b>red</b></font> and <font color='lime'><b>lime</b></font>) is shown. Superposition of the <scene name='Antizyme_Inhibitor/Azi_odc/11'>interface</scene> of mAzI and mODC showing the inter-subunit variable loops (AzI residues 355–362 and 387–401). <font color='black'><b>AzI loops</b></font> are in <font color='black'><b>black</b></font>, and <font color='black'><b>ODC loops</b></font> are in <font color='black'><b>yellow</b></font>. |
| | + | The two AzI monomers (<font color='cyan'><b>cyan</b></font>, <font color='blueviolet'><b>blueviolet</b></font>) have only <scene name='Antizyme_Inhibitor/Azi_odc1/1'>43 contacting residues</scene> (< 3.5 Å apart), while there are more contacts between the two monomers of hODC, <scene name='Antizyme_Inhibitor/Azi_odc1/2'>mODC</scene> (<font color='red'><b>red</b></font>, <font color='lime'><b>lime</b></font>), and tODC (74, 83 and 69, respectively). Moreover, the surface area buried by the two mODC monomers is significantly larger than the one buried by the AzI monomers. These features explain a very weak crystallographic AzI dimer. |
| | + | The zipper, formed by conserved [http://en.wikipedia.org/wiki/Hydrophobe hydrophobic] residues in mODC, stabilizes its dimeric structure. These residues involve F397(B), Y323(B), Y331(A), Y331(B), Y323(A), and F397(A) (the names of the chains are in brackets). The residue Y331 in the <scene name='Antizyme_Inhibitor/Azi_odc1/3'>ODC zipper</scene> is substituted by S329 in AzI and interferes with the formation of a similar zipper in AzI. Hence, in <scene name='Antizyme_Inhibitor/Azi_odc1/4'>mAzI</scene> this hydrophobic zipper is absent. Many residues, participating in the ODC interdimer interface interactions, are conserved among the ODCs from variuos organisms, but in AzI these residues are not conserved. Furthermore, the AzI conserved residues do not participate in interdimer interactions. For example, mODC possesses <scene name='Antizyme_Inhibitor/Azi_odc1/5'>two salt bridges</scene> (K169–D364 and D134–K294) stabilizing the ODC [http://en.wikipedia.org/wiki/Dimer homodimer]. In AzI, these 4 corresponding residues (<scene name='Antizyme_Inhibitor/Azi_odc1/6'>K169-D362 and D134-K291</scene>, respectively) are also present, but are too far apart to form a salt bridge. The two AzI monomers are positioned farther apart, in comparison ot ODC monomers, preventing the formation of interdimer interactions. |
| | {{Clear}} | | {{Clear}} |
| | | | |
| | + | <scene name='Antizyme_Inhibitor/Binding_site/6'>Overlap</scene> of the AzI and mODC structures suggests that AzI does not bind [http://en.wikipedia.org/wiki/Pyridoxal_phosphate PLP]. <font color='black'><b>PLP</b></font> is in <font color='black'><b>yellow</b></font>, <font color='lime'><b>ODC residues D88, R154, R277, and Y389 are in lime</b></font>, and the corresponding <font color='magenta'><b>AzI residues A88, H154, S274, and D387 are in magenta</b></font>. Many of the residues participating in PLP-ODC binding are not conserved in AzI. These include D88A, R154H, R277S, D332E, and Y389D (ODC residue numbers follow the sequence of AzI). Notably, the absence of even one of these interactions, (''e.g.'' ODC R277A mutant) results in a 100-fold decrease in PLP binding, a 50% drop in K<sub>cat</sub>, and a 7-fold decrease in K<sub>M</sub>. |
| | | | |
| - | <applet load="AzIODCali.pdb" size="500" color="white" frame="true" align="right" scene='Antizyme_Inhibitor/Azi_odc/1' spinBox="true" />
| + | ==3D structures of Antizyme Inhibitor== |
| | + | [[Antizyme inhibitor 3D structures]] |
| | | | |
| - |
| + | </StructureSection> |
| - | A sequence alignment and <scene name='Antizyme_Inhibitor/Azi_odc/10'>structural comparison</scene> of mouse AzI crystallographic dimer (mAzI, <font color='cyan'><b>cyan</b></font> and <font color='blueviolet'><b>blueviolet</b></font>) to mouse, human, and trypanosome ODC (mODC (PDB code [[7odc]], (<font color='red'><b>red</b></font> and <font color='lime'><b>lime</b></font>), hODC, and tODC, respectively) show high sequence identity (~50%) and structural similarity between AzI and ODC monomers (RMSD values of 1.85 Å, 1.6 Å, and 1.5 Å, respectively). Superposition of the <scene name='Antizyme_Inhibitor/Azi_odc/11'>interface</scene> of mAzI and mODC showing the inter-subunit variable loops (AzI residues 355–362 and 387–401). <font color='black'><b>AzI loops</b></font> are in <font color='black'><b>black</b></font>, and <font color='black'><b>ODC loops</b></font> are in <font color='black'><b>yellow</b></font>.
| + | |
| - | The two AzI monomers (<font color='cyan'><b>cyan</b></font>, <font color='blueviolet'><b>blueviolet</b></font>) have only <scene name='Antizyme_Inhibitor/Azi_odc1/1'>43 contacting residues</scene> (< 3.5 Å apart), while there are more contacts between the two monomers of hODC, mODC (<font color='red'><b>red</b></font>, <font color='lime'><b>lime</b></font>), and tODC ( <scene name='Antizyme_Inhibitor/Azi_odc1/2'>74</scene>, 83 and 69, respectively). Moreover, the surface area buried by the two mODC monomers is significantly larger than the one buried by the AzI monomers. These features explain a very weak crystallographic AzI dimer.
| + | |
| - | The zipper, formed by conserved hydrophobic residues in mODC, stabilizes its dimeric structure. These residues involve F397(B), Y323(B), Y331(A), Y331(B), Y323(A), and F397(A) (the names of the chains are in brackets). The residue Y331 in the <scene name='Antizyme_Inhibitor/Azi_odc1/3'>ODC zipper</scene> is substituted by S329 in AzI and interferes with the formation of a similar zipper in AzI. Hence, in <scene name='Antizyme_Inhibitor/Azi_odc1/4'>mAzI</scene> this hydrophobic zipper is absent. Many residues, participating in the ODC interdimer interface interactions, are conserved among the ODCs from variuos organisms, but in AzI these residues are not conserved. Furthermore, the AzI conserved residues do not participate in interdimer interactions. For example, mODC possesses <scene name='Antizyme_Inhibitor/Azi_odc1/5'>two salt bridges</scene> (K169–D364 and D134–K294) stabilizing the ODC homodimer. In AzI, these 4 corresponding residues (<scene name='Antizyme_Inhibitor/Azi_odc1/6'>K169-D362 and D134-K291</scene>, respectively) are also present, but are too far apart to form a salt bridge. The two AzI monomers are positioned farther apart, in comparison ot ODC monomers, preventing the formation of interdimer interactions.
| + | |
| - | {{Clear}}
| + | |
| | | | |
| - | <applet load="AzIODCplp.pdb" size="500" color="white" frame="true" align="right" scene='Antizyme_Inhibitor/Binding_site/5' spinBox="true" />
| + | ==Additional Resources== |
| - | | + | For additional information, see: [[Cancer]] |
| - | | + | <br /> |
| - | <scene name='Antizyme_Inhibitor/Binding_site/6'>Overlap</scene> of the AzI and mODC structures suggests that AzI does not bind PLP. <font color='black'><b>PLP</b></font> is in <font color='black'><b>yellow</b></font>, <font color='lime'><b>ODC residues D88, R154, R277, and Y389 are in lime</b></font>, and the corresponding <font color='magenta'><b>AzI residues A88, H154, S274, and D387 are in magenta</b></font>. Many of the residues participating in PLP-ODC binding are not conserved in AzI. These include D88A, R154H, R277S, D332E, and Y389D (ODC residue numbers follow the sequence of AzI). Notably, the absence of even one of these interactions, (''e.g.'' ODC R277A mutant) results in a 100-fold decrease in PLP binding, a 50% drop in K<sub>cat</sub>, and a 7-fold decrease in K<sub>M</sub>. | + | |
| - | {{Clear}}
| + | |
| | | | |
| | ==Reference== | | ==Reference== |
| Line 47: |
Line 28: |
| | [[Category: Unger, T.]] | | [[Category: Unger, T.]] |
| | [[Category: ISPC, Israel Structural Proteomics Center.]] | | [[Category: ISPC, Israel Structural Proteomics Center.]] |
| - | [[Category: Ispc]] | + | [[Category: ISPC]] |
| | [[Category: Israel structural proteomics center]] | | [[Category: Israel structural proteomics center]] |
| | [[Category: Structural genomic]] | | [[Category: Structural genomic]] |
| | + | [[Category:Topic Page]] |
|
Antizyme Inhibitor
Publication Abstract from PubMed
Antizyme inhibitor (AzI) regulates cellular polyamine homeostasis by binding to the polyamine-induced protein, Antizyme (Az), with greater affinity than ornithine decarboxylase (ODC). AzI is highly homologous to ODC but is not enzymatically active. In order to understand these specific characteristics of AzI and its differences from ODC, we determined the 3D structure of mouse AzI to 2.05 A resolution. Both AzI and ODC crystallize as a dimer. However, fewer interactions at the dimer interface, a smaller buried surface area, and lack of symmetry of the interactions between residues from the two monomers in the AzI structure suggest that this dimeric structure is nonphysiological. In addition, the absence of residues and interactions required for pyridoxal 5'-phosphate (PLP) binding suggests that AzI does not bind PLP. Biochemical studies confirmed the lack of PLP binding and revealed that AzI exists as a monomer in solution while ODC is dimeric. Our findings that AzI exists as a monomer and is unable to bind PLP provide two independent explanations for its lack of enzymatic activity and suggest the basis for its enhanced affinity toward Az.
Crystallographic and biochemical studies revealing the structural basis for antizyme inhibitor function., Albeck S, Dym O, Unger T, Snapir Z, Bercovich Z, Kahana C, Protein Sci. 2008 May;17(5):793-802. Epub 2008 Mar 27. PMID:18369191
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
Each consists of two domains: a α/β-barrel [1] domain (residues 45–280) and a modified [2] β-sheet domain (residues 8–44 and 281–435). Helices [3] are colored in red and β sheets [4] in yellow.
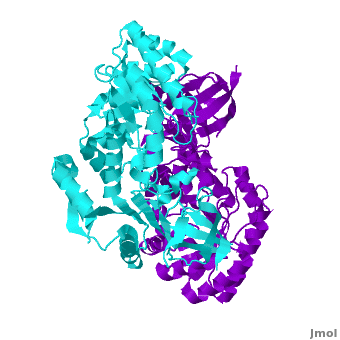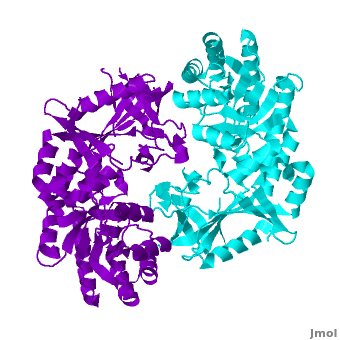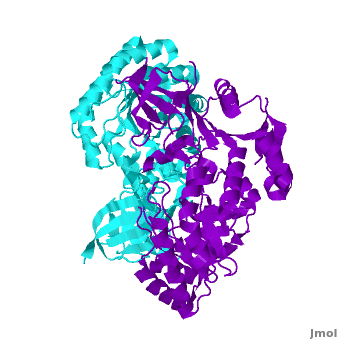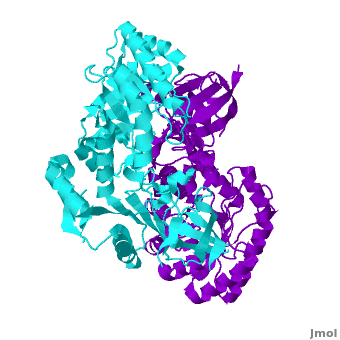
A sequence alignment and structural comparison of mouse AzI crystallographic dimer to mouse, human, and trypanosome ODC (mODC, hODC, and tODC, respectively) show high sequence identity (~50%) and structural similarity between AzI and ODC monomers (RMSD values of 1.85 Å, 1.6 Å, and 1.5 Å, respectively). The of mouse AzI crystallographic dimer (mAzI, cyan and blueviolet) to mODC (PDB code 7odc, (red and lime) is shown. Superposition of the of mAzI and mODC showing the inter-subunit variable loops (AzI residues 355–362 and 387–401). AzI loops are in black, and ODC loops are in yellow.
The two AzI monomers (cyan, blueviolet) have only (< 3.5 Å apart), while there are more contacts between the two monomers of hODC, (red, lime), and tODC (74, 83 and 69, respectively). Moreover, the surface area buried by the two mODC monomers is significantly larger than the one buried by the AzI monomers. These features explain a very weak crystallographic AzI dimer.
The zipper, formed by conserved hydrophobic residues in mODC, stabilizes its dimeric structure. These residues involve F397(B), Y323(B), Y331(A), Y331(B), Y323(A), and F397(A) (the names of the chains are in brackets). The residue Y331 in the is substituted by S329 in AzI and interferes with the formation of a similar zipper in AzI. Hence, in this hydrophobic zipper is absent. Many residues, participating in the ODC interdimer interface interactions, are conserved among the ODCs from variuos organisms, but in AzI these residues are not conserved. Furthermore, the AzI conserved residues do not participate in interdimer interactions. For example, mODC possesses (K169–D364 and D134–K294) stabilizing the ODC homodimer. In AzI, these 4 corresponding residues (, respectively) are also present, but are too far apart to form a salt bridge. The two AzI monomers are positioned farther apart, in comparison ot ODC monomers, preventing the formation of interdimer interactions.
of the AzI and mODC structures suggests that AzI does not bind PLP. PLP is in yellow, ODC residues D88, R154, R277, and Y389 are in lime, and the corresponding AzI residues A88, H154, S274, and D387 are in magenta. Many of the residues participating in PLP-ODC binding are not conserved in AzI. These include D88A, R154H, R277S, D332E, and Y389D (ODC residue numbers follow the sequence of AzI). Notably, the absence of even one of these interactions, (e.g. ODC R277A mutant) results in a 100-fold decrease in PLP binding, a 50% drop in Kcat, and a 7-fold decrease in KM.
3D structures of Antizyme Inhibitor
Antizyme inhibitor 3D structures
|
Shira Albeck, Orly Dym, Tamar Unger, Zohar Snapir, Zippy Bercovich and Chaim Kahana. Crystallographic and biochemical studies revealing the structural basis for antizyme inhibitor function. Protein Sci. 2008 May; 17(5): 793-802. Epub 2008 Mar 27.