User:Tilman Schirmer/Sandbox 201
From Proteopedia
(→Intro) |
|||
| (3 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
[[User:Tilman_Schirmer/Sandbox_200|back]] | [[User:Tilman_Schirmer/Sandbox_200|back]] | ||
| - | + | '''PleD''' | |
| - | === | + | ===Overview=== |
<applet load='1w25' scene='User:Tilman_Schirmer/Sandbox_201/Protomer/5' size='300' frame='true' align='right' caption='Diguanylate cyclase PleD (1w25)' /> | <applet load='1w25' scene='User:Tilman_Schirmer/Sandbox_201/Protomer/5' size='300' frame='true' align='right' caption='Diguanylate cyclase PleD (1w25)' /> | ||
| Line 29: | Line 29: | ||
| - | The motif is part of the <scene name='User:Tilman_Schirmer/Sandbox_201/Substrate_binding_site/ | + | The motif is part of the <scene name='User:Tilman_Schirmer/Sandbox_201/Substrate_binding_site/5'>substrate binding site</scene> as identified in the structure of PleD in complex with <scene name='User:Tilman_Schirmer/Sandbox_201/Gtp-a-s/2'>GTP-alpha-S / Mg++</scene>. The GGDEFY domain binds only '''one''' GTP subsrate molecule. For the reaction to proceed, '''two''' GTP loaded GGDEF domains have to align antiparallely. MODEL. |
| Line 36: | Line 36: | ||
<br><br><br><br><br><br><br><br><br><br><br><br><br> | <br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
| - | == Allosteric product binding site == | + | === Allosteric product binding site === |
<applet load='2v0n' scene='User:Tilman_Schirmer/Sandbox_201/5gp/1' size='300' frame='true' align='right' caption='Allosteric product binding site' /> | <applet load='2v0n' scene='User:Tilman_Schirmer/Sandbox_201/5gp/1' size='300' frame='true' align='right' caption='Allosteric product binding site' /> | ||
Current revision
PleD
Contents |
Overview
|
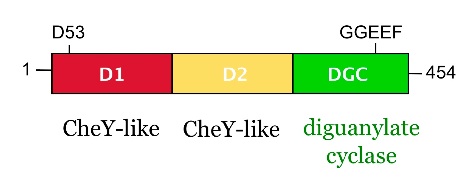
from Caulobacter crescentus is a response regulator with an unorthodox catalytic, diguanylate cyclase, output domain. It is composed of a canonical CheY-like response regulator receiver () domain,
a Rec-like () adaptor domain,
and a C-terminal domain that confers the catalytic acitvity.
The GGDEF domain is named after the highly conserved (in PleD it is GGEEF) that locates to a β-hairpin.
Substrate binding
|
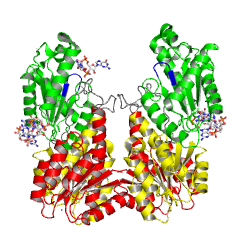
The motif is part of the as identified in the structure of PleD in complex with . The GGDEFY domain binds only one GTP subsrate molecule. For the reaction to proceed, two GTP loaded GGDEF domains have to align antiparallely. MODEL.
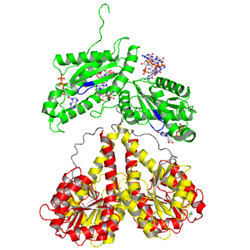
Allosteric product binding site
|
C-di-GMP
Primary inhibition site (Ip)
Secondary inhibition site (Is)
Primary and secondary inhibition sites
Two conformations
|
|