Ann taylor sandbox 5
From Proteopedia
(New page: {{Seed}} 200px <!-- The line below this paragraph, containing "STRUCTURE_3d06", creates the "Structure Box" on the page. You may change the PDB parameter (which se...) |
|||
| (9 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | {{ | + | {{STRUCTURE_2ada| PDB=2ada | SCENE= }} |
| - | + | ||
| - | + | ===ADENOSINE DEAMINASE=== | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | ===Human p53 core domain with hot spot mutation R249S (I)=== | ||
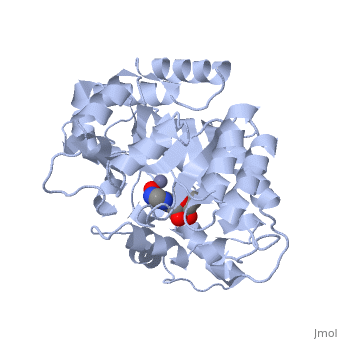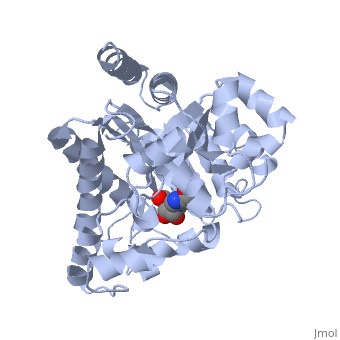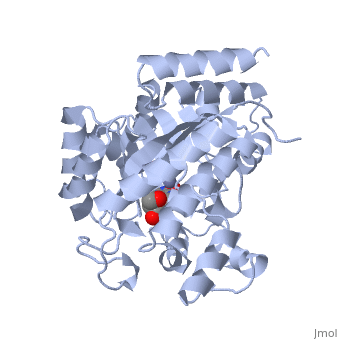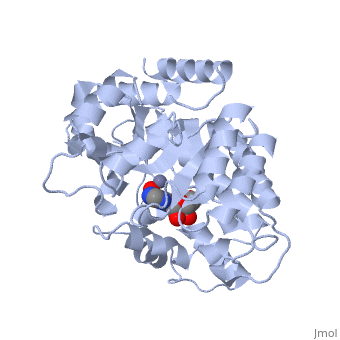
| + | Adenosine deaminase is involved in the degradation of purine nucleotides. It is especially active in lympocytes, and mutation of adenosine deaminase results in severe immunodeficiency. Adenosine deaminase contains an eight stranded parallel alpha/beta barrel with the active site in a deep pocket at the beta-barrel COOH-terminal end. <ref>PMID:1925539 </ref> The active site contains a <scene name='36/365337/Zinc_cofactor/6'>Zinc cofactor</scene>, which coordinates to the 6-hydroxyl of the transition state analogue, 6-hydroxyl, 1,6-dihydropurine ribonucleoside. The zinc is coordinated to <scene name='36/365337/Histidine_residues/2'>three histidine residues</scene> and an <scene name='36/365337/Aspartic_acid_residue/1'>aspartic acid residue</scene>. | ||
| - | + | The transition state analogue held in place mostly by polar interactions. The ribose group is close to the opening of the pocket, with the purine portion deeper in the pocket, close to the zinc. Nine hydrogen bonds stabilize the transition state-enzyme complex. | |
| - | The | + | |
| - | + | ||
| - | - | + | |
| - | + | ||
| - | + | ADA is very stereoselective for the 6R isomer. This specificity is due to the location of the catalytic zinc, Asp295 and His 238. Interestingly, one face of the purine ring is exposed to polar groups and zinc, while the other face is only exposed to nonpolar residues. The proposed catalytic mechanism has Asp295 act as a general base, while the zinc acts as an electrophile to activate the water molecule. His 238 orients the water and stabilizes the charge of the attacking hydroxide. The protonated <scene name='36/365337/Glu217/1'>Glu217</scene> or the water hydrogen bonded to it could donate or share a proton with the N1 of the substrate. | |
| - | + | ||
| - | ==About this Structure== | ||
| - | 3D06 is a 1 chain structure of sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=3D06 OCA]. | ||
| - | + | <references/> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Current revision
| |||||||||
| 2ada, resolution 2.40Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||||
| Activity: | Adenosine deaminase, with EC number 3.5.4.4 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
ADENOSINE DEAMINASE
Adenosine deaminase is involved in the degradation of purine nucleotides. It is especially active in lympocytes, and mutation of adenosine deaminase results in severe immunodeficiency. Adenosine deaminase contains an eight stranded parallel alpha/beta barrel with the active site in a deep pocket at the beta-barrel COOH-terminal end. [1] The active site contains a , which coordinates to the 6-hydroxyl of the transition state analogue, 6-hydroxyl, 1,6-dihydropurine ribonucleoside. The zinc is coordinated to and an .
The transition state analogue held in place mostly by polar interactions. The ribose group is close to the opening of the pocket, with the purine portion deeper in the pocket, close to the zinc. Nine hydrogen bonds stabilize the transition state-enzyme complex.
ADA is very stereoselective for the 6R isomer. This specificity is due to the location of the catalytic zinc, Asp295 and His 238. Interestingly, one face of the purine ring is exposed to polar groups and zinc, while the other face is only exposed to nonpolar residues. The proposed catalytic mechanism has Asp295 act as a general base, while the zinc acts as an electrophile to activate the water molecule. His 238 orients the water and stabilizes the charge of the attacking hydroxide. The protonated or the water hydrogen bonded to it could donate or share a proton with the N1 of the substrate.