AChE inhibitors and substrates (Part II)
From Proteopedia
| Line 27: | Line 27: | ||
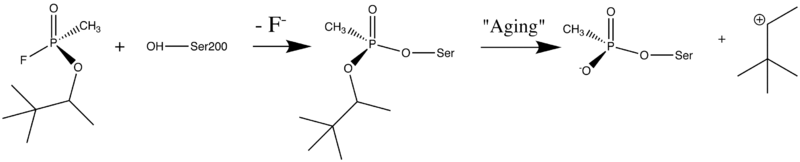
[[Image:Soman_reaction.png | left | thumb | 800px | Reaction between Ser200OG and Soman, assuming an in-line attack by the OG, followed by spontaneous dealkylation of the O-pinacolyl group.]] | [[Image:Soman_reaction.png | left | thumb | 800px | Reaction between Ser200OG and Soman, assuming an in-line attack by the OG, followed by spontaneous dealkylation of the O-pinacolyl group.]] | ||
<br style="clear:both;"/> | <br style="clear:both;"/> | ||
| + | {{Clear}} | ||
| + | <applet load='Soman1.pdb' size='500' frame='true' align='right' scene='2wfz/Al/1' /> | ||
| + | Acetylcholinesterase (AChE) hydrolysizes the neurotransmitter <scene name='2wfz/Al/2'>acetylcholine (ACh)</scene>, producing <scene name='2wfz/Al/3'>choline and an acetate</scene> group. <scene name='2wfz/Al/2'>ACh</scene> directly binds catalytic <scene name='2wfz/Al/4'>Ser200</scene> (via its nucleophilic Oγ atom). <scene name='2wfz/Al/5'>Soman</scene>, O-(1,2,2-trimethylpropyl) methylphosphonofluoridate (<font color='violet'><b>fluorine atom is colored violet</b></font> and <font color='darkmagenta'><b>phosphorus atom is colored darkmagenta</b></font>), is one of the most toxic organophosphate compounds (OPs). Soman inhibits AChE by <scene name='2wfz/Al/6'>covalent binding</scene> to catalytic Ser200, <scene name='2wfz/Al/7'>mimicking ACh</scene>. This process <scene name='2wfz/Al/8'>(phosphonylation)</scene> implicates nucleophilic attack of the Ser200 nucleophilic Oγ atom on the phosphorus atom of soman, with concomitant departure of its fluoride atom. After that AChE catalyzes the <scene name='2wfz/Al/9'>dealkylation ("aging")</scene> of the soman or other OP. This causes irreversible inhibition of AChE, "aged" soman/AChE conjugate can not be reactivated. However, before “aging”, at the step of <scene name='2wfz/Al/8'>phosphonylation</scene>, AChE can be <scene name='2wfz/Al/11'>reactivated</scene> by nucleophiles, such as pralidoxime (2-PAM), resulting in <scene name='2wfz/Al/12'>cleavage</scene> of the phosphonyl adduct from Ser200 Oγ. | ||
| + | At the <scene name='2wfz/Ali/3'>active site of the nonaged soman/TcAChE conjugate</scene> ('''2wfz''') the catalytic His440 forms hydrogen bonds with Ser200 Oγ and Glu327 Oε1 via its Nε2 and Nδ1 nitrogens, respectively. The O2 atom of soman is within hydrogen bonding distance of His440 Nε2. Soman O1 mimicks carbonyl oxygen of ACh. A water molecule 1001 interacting with soman O2 is represented as a <font color='red'><b>red ball</b></font>. The active site residues of the nonaged soman/TcAChE are colored <font color='yellow'><b>yellow</b></font>. The O2 atom of the <scene name='2wfz/Ali/4'>dealkylated (aged) soman</scene> ([[2wg0]]) forms a salt bridge with His440 Nε2. The active site residues of the aged soman/TcAChE are colored <font color='pink'><b>pink</b></font>. <scene name='2wfz/Ali/5'>Alignment</scene> of the structures of the nonaged ('''2wfz''') and aged ([[2wg0]]) conjugates reveals a small, but important, change within the active site - the imidazole ring of His440 is tilted back to a native-like conformation after dealkylation. The water molecule 1001, which interacts with soman O2 in the nonaged crystal structure, is not within hydrogen bonding distance of O2 in the aged crystal structure. 2-PAM binds poorly to the nonaged phosphonylated enzyme (its electron density was not found) and binds in an <scene name='2wfz/Ali/7'>unfavorable and nonfunctional conformation</scene> after soman aging to ''Tc''AChE ([[2wg1]]). | ||
| + | |||
| + | {{Clear}} | ||
| + | |||
<br /><applet load="1som" size="350" color="white" frame="true" align="right" spinBox="true" | <br /><applet load="1som" size="350" color="white" frame="true" align="right" spinBox="true" | ||
caption="1som, resolution 2.20Å" scene="1som/Ache_soman/1"/> <br /> | caption="1som, resolution 2.20Å" scene="1som/Ache_soman/1"/> <br /> | ||
Revision as of 15:51, 1 December 2009
AChE monovalent inhibitors (continuation of the page AChE inhibitors and substrates)
|
6) Edrophonium (EDR) is stacked between the aromatic rings of , near the TcAChE which consists of S200, E327, and H440 (2ack).
|
7) Rivastigmine (Exelon) is a carbamate inhibitor of AChE, and it is currenly used in therapy of Alzheimer's disease. Rivastigmine (colored yellow) interacts with TcAChE (colored lime) at the . The carbamyl moiety of rivastigmine is to the active-site S200 Oγ. The second part of rivastigmine (the leaving group), NAP ((−)-S-3-[1-(dimethylamino)ethyl]phenol) is also held in the active-site gorge, but it is from the carbamyl moiety, hence, carbamylation took place. The of TcAChE/NAP (colored magenta) is known (1gqs). The TcAChE active-site residues which are interacting with NAP are colored violet. NAP is located in a similar region of TcAChE active site, but with different orientation than that of the NAP part (colored yellow) in the TcAChE/rivastigmine complex. Only H440 and F330 significantly change their side-chain conformations. of the TcAChE active sites in 4 different structures (TcAChE/rivastigmine, TcAChE/NAP, native TcAChE (2ace), and TcAChE/VX (1vxr, TcAChE colored white and VX black) reveals that the conformation of H440 in the TcAChE/NAP structure is very similar its conformation in the native TcAChE (2ace), but the distance between H440 Nδ and E327 Oε is significantly longer in the TcAChE/rivastigmine and the TcAChE/VX complexes. This structural change disrupts the catalytic triad consisting of S200, E327, H440. This could explain the very slow kinetics of AChE reactivation after its inhibition by rivastigmine.
|
8) The TcAChE active site consists of two binding subsites. One of them is the "catalytic anionic site" (CAS), which involves the catalytic triad (colored orange) and the conserved residues which also participate in ligand recognition. Another conserved residue (colored cyan) is situated at the second binding subsite, termed the "peripheral anionic site" (PAS), ~14 Å from CAS. is a good example of the PAS-binding AChE inhibitors. of the crystal structure of the edrophonium/TcAChE (mentioned above as a CAS-binding inhibitor) (2ack) on the thioflavin T/TcAChE complex structure (2j3q) shows that these ligands' positions do not overlap. Of note is that Phe330, which is part of the CAS, is the single residue interacting with thioflavin T. This residue is the only one which significantly to avoid clashes in comparison to other CAS residues of the edrophonium/TcAChE complex.
9) Organophosphorus acid anhydride (OP) nerve agents are potent inhibitors which rapidly phosphonylate AChE and then may undergo an internal dealkylation reaction (called "aging") to produce an OP-enzyme conjugate that cannot be reactivated.
|
Acetylcholinesterase (AChE) hydrolysizes the neurotransmitter , producing group. directly binds catalytic (via its nucleophilic Oγ atom). , O-(1,2,2-trimethylpropyl) methylphosphonofluoridate (fluorine atom is colored violet and phosphorus atom is colored darkmagenta), is one of the most toxic organophosphate compounds (OPs). Soman inhibits AChE by to catalytic Ser200, . This process implicates nucleophilic attack of the Ser200 nucleophilic Oγ atom on the phosphorus atom of soman, with concomitant departure of its fluoride atom. After that AChE catalyzes the of the soman or other OP. This causes irreversible inhibition of AChE, "aged" soman/AChE conjugate can not be reactivated. However, before “aging”, at the step of , AChE can be by nucleophiles, such as pralidoxime (2-PAM), resulting in of the phosphonyl adduct from Ser200 Oγ. At the (2wfz) the catalytic His440 forms hydrogen bonds with Ser200 Oγ and Glu327 Oε1 via its Nε2 and Nδ1 nitrogens, respectively. The O2 atom of soman is within hydrogen bonding distance of His440 Nε2. Soman O1 mimicks carbonyl oxygen of ACh. A water molecule 1001 interacting with soman O2 is represented as a red ball. The active site residues of the nonaged soman/TcAChE are colored yellow. The O2 atom of the (2wg0) forms a salt bridge with His440 Nε2. The active site residues of the aged soman/TcAChE are colored pink. of the structures of the nonaged (2wfz) and aged (2wg0) conjugates reveals a small, but important, change within the active site - the imidazole ring of His440 is tilted back to a native-like conformation after dealkylation. The water molecule 1001, which interacts with soman O2 in the nonaged crystal structure, is not within hydrogen bonding distance of O2 in the aged crystal structure. 2-PAM binds poorly to the nonaged phosphonylated enzyme (its electron density was not found) and binds in an after soman aging to TcAChE (2wg1).
|
To understand the basis for irreversible inhibition, the structure of the aged conjugate obtained by reaction of Torpedo californica AChE (TcAChE) with O-pinacolylmethylphosphonofluoridate (soman) was solved by X-ray crystallography to 2.2Å resolution (1som). The highest positive difference density peak corresponded to the OP phosphorus and was located within covalent bonding distance of the active-site serine (S200). The are within hydrogen-bonding distance of four potential donors from catalytic subsites of the enzyme, suggesting that electrostatic forces significantly stabilize the aged enzyme. The methyl group of soman occupies the , bounded by Trp233, Phe288, and Phe290. The active sites of aged sarin-TcAChE (1cfj) and soman-TcAChE were essentially identical and provided structural models for the negatively charged, tetrahedral intermediate that occurs during deacylation with the natural substrate, acetylcholine.
|
10) is a nonhydrolyzable substrate analogue of AChE. Its hydrolysis is impossible as OTMA possesses atom instead of the in the AChE natural substrate ACh. Similarly to ACh, OTMA covalently binds to the TcAChE (2vja) Oγ at the CAS. At this subsite OTMA also interacts with (cation-π interactions); (electrostatic interaction); (hydrogen bonds). OTMA binds not only at CAS, but also at PAS. A second OTMA molecule interacts with (cation-π interactions), and (weak hydrogen bond). Thus, this dual binding mode of OTMA with TcAChE (to CAS and PAS) could be prototypical for AChE bivalent inhibitors.
Please see also our pages AChE bivalent inhibitors and AChE bivalent inhibitors (Part II).
Selected 3D Structures of AChE
- 2ace This is the original solved structure for Torpedo Californica
- 1ea5 This is one of the highest quality representative X-ray structures in the PDB.
- 1eve The E2020 (Aricept) complex.
- 1ax9 Endrophonium complex.
- 1vot Complex with Huperzine, a Chinese folk medicine.
- 1fss Complex with snake venum toxin Fasciculin-II.
- 1acj Complex with tacrine.
- 1e66 Complex with huprine X.
- 1dx6 Complex with galanthamine.
- 1w6r Complex with galanthamine iminium derivative.
- 2ack Complex with edrophonium.
- 1vzj Structure of the tetramerization domain of acetylcholinesterase.
- 1gqr Complex with rivastigmine.
- 1gqs Complex with NAP alone.
- 1vxr Complex with VX.
- 2vja Complex with OTMA.
- 1som Complex with soman.
- 1cfj Complex with sarin
References
- Raves ML, Harel M, Pang YP, Silman I, Kozikowski AP, Sussman JL. Structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A. Nat Struct Biol. 1997 Jan;4(1):57-63. PMID:8989325
- Harel M, Schalk I, Ehret-Sabatier L, Bouet F, Goeldner M, Hirth C, Axelsen PH, Silman I, Sussman JL. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9031-5. PMID:8415649
- Greenblatt HM, Guillou C, Guenard D, Argaman A, Botti S, Badet B, Thal C, Silman I, Sussman JL. The complex of a bivalent derivative of galanthamine with torpedo acetylcholinesterase displays drastic deformation of the active-site gorge: implications for structure-based drug design. J Am Chem Soc. 2004 Dec 1;126(47):15405-11. PMID:15563167 doi:http://dx.doi.org/10.1021/ja0466154
- Ravelli RB, Raves ML, Ren Z, Bourgeois D, Roth M, Kroon J, Silman I, Sussman JL. Static Laue diffraction studies on acetylcholinesterase. Acta Crystallogr D Biol Crystallogr. 1998 Nov 1;54(Pt 6 Pt 2):1359-66. PMID:10089512
- Haviv H, Wong DM, Greenblatt HM, Carlier PR, Pang YP, Silman I, Sussman JL. Crystal packing mediates enantioselective ligand recognition at the peripheral site of acetylcholinesterase. J Am Chem Soc. 2005 Aug 10;127(31):11029-36. PMID:16076210 doi:http://dx.doi.org/10.1021/ja051765f
- Carlier PR, Du DM, Han Y, Liu J, Pang YP. Potent, easily synthesized huperzine A-tacrine hybrid acetylcholinesterase inhibitors. Bioorg Med Chem Lett. 1999 Aug 16;9(16):2335-8. PMID:10476864
- Wong DM, Greenblatt HM, Dvir H, Carlier PR, Han YF, Pang YP, Silman I, Sussman JL. Acetylcholinesterase complexed with bivalent ligands related to huperzine a: experimental evidence for species-dependent protein-ligand complementarity. J Am Chem Soc. 2003 Jan 15;125(2):363-73. PMID:12517147 doi:http://dx.doi.org/10.1021/ja021111w
- Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872-9. PMID:1678899
- Greenblatt HM, Kryger G, Lewis T, Silman I, Sussman JL. Structure of acetylcholinesterase complexed with (-)-galanthamine at 2.3 A resolution. FEBS Lett. 1999 Dec 17;463(3):321-6. PMID:10606746
- Bar-On P, Millard CB, Harel M, Dvir H, Enz A, Sussman JL, Silman I. Kinetic and structural studies on the interaction of cholinesterases with the anti-Alzheimer drug rivastigmine. Biochemistry. 2002 Mar 19;41(11):3555-64. PMID:11888271
- Colletier JP, Bourgeois D, Sanson B, Fournier D, Sussman JL, Silman I, Weik M. Shoot-and-Trap: use of specific x-ray damage to study structural protein dynamics by temperature-controlled cryo-crystallography. Proc Natl Acad Sci U S A. 2008 Aug 19;105(33):11742-7. Epub 2008 Aug 13. PMID:18701720
- Dvir H, Wong DM, Harel M, Barril X, Orozco M, Luque FJ, Munoz-Torrero D, Camps P, Rosenberry TL, Silman I, Sussman JL. 3D structure of Torpedo californica acetylcholinesterase complexed with huprine X at 2.1 A resolution: kinetic and molecular dynamic correlates. Biochemistry. 2002 Mar 5;41(9):2970-81. PMID:11863435
- Harel M, Sonoda LK, Silman I, Sussman JL, Rosenberry TL. Crystal structure of thioflavin T bound to the peripheral site of Torpedo californica acetylcholinesterase reveals how thioflavin T acts as a sensitive fluorescent reporter of ligand binding to the acylation site. J Am Chem Soc. 2008 Jun 25;130(25):7856-61. Epub 2008 May 31. PMID:18512913 doi:http://dx.doi.org/10.1021/ja7109822
- Millard CB, Kryger G, Ordentlich A, Greenblatt HM, Harel M, Raves ML, Segall Y, Barak D, Shafferman A, Silman I, Sussman JL. Crystal structures of aged phosphonylated acetylcholinesterase: nerve agent reaction products at the atomic level. Biochemistry. 1999 Jun 1;38(22):7032-9. PMID:10353814 doi:http://dx.doi.org/10.1021/bi982678l
Proteopedia Page Contributors and Editors (what is this?)
Alexander Berchansky, Wayne Decatur, David Canner, Michal Harel