Ann Taylor sandbox 20
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | == | + | == Your Heading Here (maybe something like 'Structure') == |
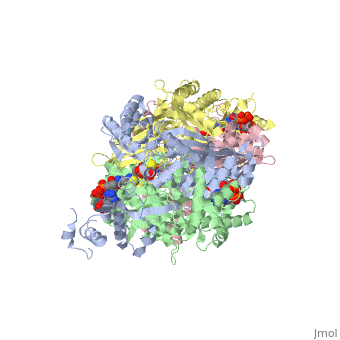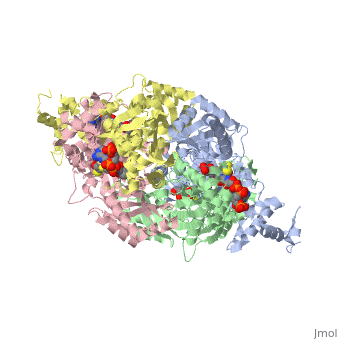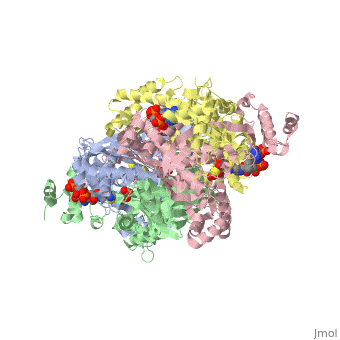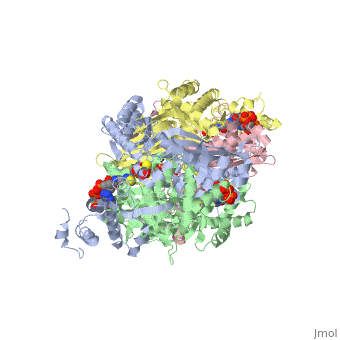
| - | + | <StructureSection load='1dq8' size='350' side='right' caption='Structure of HMG-CoA reductase (PDB entry [[1dq8]])' scene=''> | |
| - | + | Anything in this section will appear adjacent to the 3D structure and will be scrollable. | |
| - | + | This sandbox is for use by a BCMB 402 student. | |
| - | + | </StructureSection> | |
| - | + | ||
| - | < | + | |
Current revision
Your Heading Here (maybe something like 'Structure')
| |||||||||||