Anthony Noles Sandbox
From Proteopedia
| Line 14: | Line 14: | ||
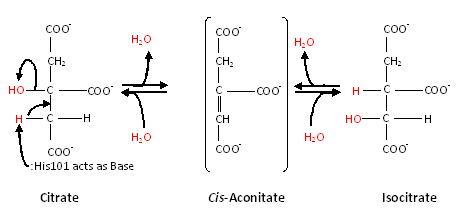
Thus, the overall reaction that aconitase catalyzes is: Citrate ←→cis-Aconitate←→Isocitrate, as seen below: | Thus, the overall reaction that aconitase catalyzes is: Citrate ←→cis-Aconitate←→Isocitrate, as seen below: | ||
| - | [[Image: | + | [[ Image:Aconitase.JPG]] |
==References== | ==References== | ||
Revision as of 17:42, 1 March 2010
Contents |
Aconitase
Aconitase (PDB 7acn) catalyzes the reversible isomerization of citrate and isocitrate. It is the second enzyme is the Citric acid cycle. The consists of numerous alternating alpha helices and beta sheets (α/β alternating). The tertiary structure is somewhat bilobed with the active site in the middle, and, since there is only one subunit, there is no quaternary structure.
Mechanism of Aconitase
| |||||||||
| 7acn, resolution 2.00Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||||
| Non-Standard Residues: | |||||||||
| Activity: | Aconitate hydratase, with EC number 4.2.1.3 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Aconitase contains . This iron sulfur cluster does not participate in redox as most do, but coordinates the OH goup of citrate to facilitate its elimination.[1] It is as this 4Fe-4S site that catalysis occurs and citrate or is bound. The rest of the is made up of residues Gln72, Asp100, His101, Asp165, Ser166, His167, His147, Glu262, Asn258, Cys358, Cys421, Cys424, Cys358, Cys421, Asn446, Arg447, Arg452, Asp568, Ser642, Ser643, Arg644, Arg580. [2]
Substrate-free aconitase contains a [4Fe-4S]2+ cluster with hydroxyl bound to one of the Fe. Upon binding of substrate the bound hydroxyl is protonated. A hydrogen bond from to the isocitrate hydroxyl is donated to form water. Alternatively, the proton could be donated by as this histidine is hydrogen bonded to a H2O molecule. His167 is also hydrogen bonded to the bound H2O in the [4Fe-4S] cluster. Both are paired with carboxylates (, respectively) and are likely to be protonated. The conformational change associated with substrate binding which reorients the cluster. [3] The residue which abstracts a proton from the alpha carbon of citrate or isocitrate is . [4] This causes the cis-Aconitate intermediate (seen below), which consists of a double bond, which is a direct result of the deprotonation. Then, there is a rehydration of the double bond of cis-aconitate to form isocitrate (if the original substrate was citrate). To better understand this, consider this process as stages, seen below.
Stage 1: Dehydration
First, dehydration of citrate causes a proton and OH group to be removed from only the 'lower arm'.[5] This forms a cis-Aconitate intermediate.
Stage 2: Rehydration
The second main stage of the reaction is the rehydration of the cis-Aconitate intermediate. This forms isocitrate. It is catalyzed in a stereospecific way such that only one isocitrate stereoisomer is formed. [6]
Thus, the overall reaction that aconitase catalyzes is: Citrate ←→cis-Aconitate←→Isocitrate, as seen below:
References
- ↑ Dupuy J, Volbeda A, Carpentier P, Darnault C, Moulis JM, Fontecilla-Camps JC. Crystal structure of human iron regulatory protein 1 as cytosolic aconitase. Structure. 2006 Jan;14(1):129-39. PMID:16407072 doi:10.1016/j.str.2005.09.009
- ↑ Beinert, H., Kennedy, M. C., Stout, C.D. “Aconitase as Iron−Sulfur Protein, Enzyme, and Iron-Regulatory Protein.” Chem. Rev. 1996, 96, 2335−2373.
- ↑ Beinert, H., Kennedy, M. C., Stout, C.D. “Aconitase as Iron−Sulfur Protein, Enzyme, and Iron-Regulatory Protein.” Chem. Rev. 1996, 96, 2335−2373.
- ↑ Beinert, H., Kennedy, M. C., Stout, C.D. “Aconitase as Iron−Sulfur Protein, Enzyme, and Iron-Regulatory Protein.” Chem. Rev. 1996, 96, 2335−2373.
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry Life at the Molecular Level. New York: John Wiley & Sons, 2008. p. 578. Print.
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry Life at the Molecular Level. New York: John Wiley & Sons, 2008. p. 579. Print.

