Pyruvate Kinase
From Proteopedia
| Line 3: | Line 3: | ||
==Mechanism== | ==Mechanism== | ||
| - | Pyruvate | + | <scene name='Keegan_Gelvoria_Sandbox_1/N_c_rainbow/null'>Pyruvate Kinase</scene> catalyzes the final reaction of glycolysis. It couples the free energy of PEP cleavage to the generation of ATP during the synthesis of the final product, pyruvate. This reaction necessitates both K+ and Mg2+ cations, which has two steps. The first step is the nucleophilic attack of the PEP phosphorous atom by β-phosphoryl oxygen of ADP; this step displaces enolpyruvate while forming ATP. In the second step, enolpyruvate tautomerizes to pyruvate. The formation of a high-energy intermediate by enolase in the 9th reaction of glycolysis allows for the synthesis of ATP in this reaction. Though the hydrolysis of 2PG is insufficient in driving the synthesis of ATP, the dehydration of 2PG allows for such a reaction to occur by forming a high-energy intermediate. The high potential of PEP reflects the large release of energy that occurs with the conversion of enolpyruvate to its keto tautomer, pyruvate. |
==Structure== | ==Structure== | ||
| Line 18: | Line 18: | ||
Species: Human (Homo sapiens) | Species: Human (Homo sapiens) | ||
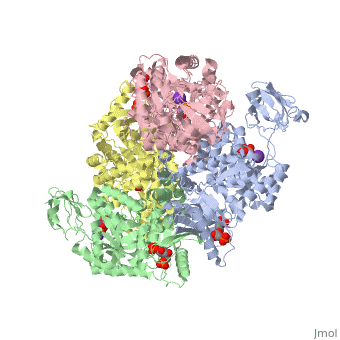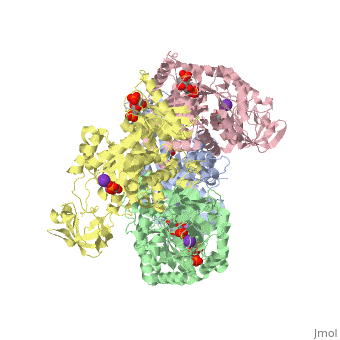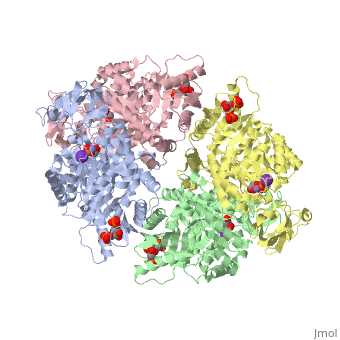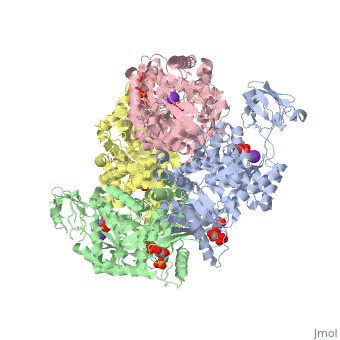
| + | As can be seen, pyruvate kinase's <scene name='Keegan_Gelvoria_Sandbox_1/Secondary_structure/1'>secondary structure</scene> comprises of <scene name='Keegan_Gelvoria_Sandbox_1/Structure_4_domains/1'>four domains</scene> in humans. Thus, this enzyme is tetrameric with <scene name='Keegan_Gelvoria_Sandbox_1/Metal_binding_sites/1'>metal binding sites</scene> on each domain for the K+ and Mg2+ ligands to bind to. There are four types of tissue-specific isozymes: L (liver), R (red blood cells), M1 (muscle, heart, and brain), and M2 (early fetal tissue). | ||
Replace the PDB id (use lowercase!) after the STRUCTURE_ and after PDB= to load | Replace the PDB id (use lowercase!) after the STRUCTURE_ and after PDB= to load | ||
Revision as of 21:34, 1 March 2010
Pyruvate Kinase
Pyruvate kinase is an enzyme that is involved in glycolysis. Pyruvate kinase’s function is to catalyze the last step of glycolysis; thereby, generating the second ATP of glycolysis and pyruvate. It is able to catalyze this step by transferring the phosphate group from phosphoenolpyruvate (PEP) to ADP.
Mechanism
catalyzes the final reaction of glycolysis. It couples the free energy of PEP cleavage to the generation of ATP during the synthesis of the final product, pyruvate. This reaction necessitates both K+ and Mg2+ cations, which has two steps. The first step is the nucleophilic attack of the PEP phosphorous atom by β-phosphoryl oxygen of ADP; this step displaces enolpyruvate while forming ATP. In the second step, enolpyruvate tautomerizes to pyruvate. The formation of a high-energy intermediate by enolase in the 9th reaction of glycolysis allows for the synthesis of ATP in this reaction. Though the hydrolysis of 2PG is insufficient in driving the synthesis of ATP, the dehydration of 2PG allows for such a reaction to occur by forming a high-energy intermediate. The high potential of PEP reflects the large release of energy that occurs with the conversion of enolpyruvate to its keto tautomer, pyruvate.
Structure
Class: All Beta Proteins
Fold: PK beta-barrel domain-like
Superfamily: PK beta-barrel domain-like
Family: Pyruvate kinase beta-barrel domain
Protein: Pyruvate Kinase (PK)
Species: Human (Homo sapiens)
As can be seen, pyruvate kinase's comprises of in humans. Thus, this enzyme is tetrameric with on each domain for the K+ and Mg2+ ligands to bind to. There are four types of tissue-specific isozymes: L (liver), R (red blood cells), M1 (muscle, heart, and brain), and M2 (early fetal tissue).
Replace the PDB id (use lowercase!) after the STRUCTURE_ and after PDB= to load and display another structure.
| |||||||||
| 2vgb, resolution 2.73Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , , | ||||||||
| Activity: | Pyruvate kinase, with EC number 2.7.1.40 | ||||||||
| Related: | 1liy, 1liu, 1liw, 1lix | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Keegan Gelvoria, David Canner, Ann Taylor, Andrew Alexander