Prolyl Endopeptidase
From Proteopedia
| Line 12: | Line 12: | ||
=== Catalytic Domain === | === Catalytic Domain === | ||
| - | |||
| - | === Domain Interface === | ||
| Line 21: | Line 19: | ||
PEPs are thought to have a role in the degradation of [http://en.wikipedia.org/wiki/Neuropeptides neuropeptides] due to the high concentration of PEPs in the brain and the fact that PEPs have been shown to degrade several [http://en.wikipedia.org/wiki/Neuropeptides neuropeptides](vasopressin, β-endorphin, thyroliberin). The distribution of PEP in the brain has been found to be similar to that of certain receptors of [http://en.wikipedia.org/wiki/Neuropeptides neuropeptides] which supports PEPs being involved in the degradation of [http://en.wikipedia.org/wiki/Neuropeptides neuropeptide] transmitters. | PEPs are thought to have a role in the degradation of [http://en.wikipedia.org/wiki/Neuropeptides neuropeptides] due to the high concentration of PEPs in the brain and the fact that PEPs have been shown to degrade several [http://en.wikipedia.org/wiki/Neuropeptides neuropeptides](vasopressin, β-endorphin, thyroliberin). The distribution of PEP in the brain has been found to be similar to that of certain receptors of [http://en.wikipedia.org/wiki/Neuropeptides neuropeptides] which supports PEPs being involved in the degradation of [http://en.wikipedia.org/wiki/Neuropeptides neuropeptide] transmitters. | ||
| - | PEPs may also have a more general role in the degradation of peptides as PEP activity is found in most major organs and many peptidases cannot cleave proline residues. | + | PEPs may also have a more general role in the degradation of peptides as PEP activity is found in most major organs and many other peptidases cannot cleave proline residues. |
=== Binding Mechanism === | === Binding Mechanism === | ||
Revision as of 21:57, 25 March 2010
|
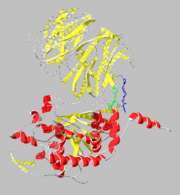
Prolyl endopeptidases (PEPs) are a class of serine proteases which cleave peptide bonds on the C-terminal side of internal proline residues.
Contents |
Structure
Prolyl endopeptidases are relatively large enzymes(75 kDa) and contain two distinct domains: a catalytic domain and a β-propeller domain.
β-Propeller Domain
The β-propeller domain is made up of repeated antiparallel β-sheets forming a tight lid over the active site located on the catalytic domain. This propeller domain is proposed to be very important in allowing the binding of subtrate as well as the inability of PEPs to hydrolyze peptide chains longer than 30 amino acids.
Catalytic Domain
Function
Althought the physiological function of PEPs are not entirely understood there are several main proposed functions based on their activity and localization.
PEPs are thought to have a role in the degradation of neuropeptides due to the high concentration of PEPs in the brain and the fact that PEPs have been shown to degrade several neuropeptides(vasopressin, β-endorphin, thyroliberin). The distribution of PEP in the brain has been found to be similar to that of certain receptors of neuropeptides which supports PEPs being involved in the degradation of neuropeptide transmitters.
PEPs may also have a more general role in the degradation of peptides as PEP activity is found in most major organs and many other peptidases cannot cleave proline residues.
Binding Mechanism
Inhibition
Pharmaceutical Possibilities
Both human and microbial PEP have been the focus of research into their viability as therapeutic agents for several diseases. The ability of PEPs to cleave internal proline peptide bonds is of interest in the treatment of Celiac Disease which is caused by a reaction to gluten, a proline rich protein found in wheat and wheat subspecies. CITE BESEDIN PEPs have also been linked to several neurological disorders due to high activity in the brain and proposed role in the degradation of neuropeptides.
Celiac Disease
Celiac Disease(CD) is a genetic disorder marked by diarrhea, fatigue, weight loss, and villous atrophy due to the consumption of certain proteins such as gluten and other prolamins found in wheat and wheat subspecies. The symptoms of CD are due to an auto-immune inflammatory reaction that occurs in the small intestine due to prolamins that are rich in proline residues and known to be resistant to many proteases.
There is currently no cure for Celiac Disease and the main treatment is to simply avoid eating prolamin containing foods.
Prolyl endopeptidases are a promising therapeutic agent due to their ability to degrade the protein rich prolamins and inhibit the inflammatory reaction. An early idea for treatment is to orally administer certain microbial PEPs which could directly degrade prolamins in the intestine and reduce the auto-immune response. The key for this possibility is to be able to protect the PEPs from being degraded by gastric acids while allowing them to be able to be functional in the small intestine once the acidity is decreased.
Research by Ehren, Govindarajan, Morón, Minshull, and Khosla CITE has shown the ability to engineer the prolyl endopeptidase of Sphingomonas capsulata to increase the activity of this PEP under simulated gastric conditions showing the relevance of PEPs as a potential therapeutic agent for Celiac Disease.
Neurological Disorders
References
[] Ehren J, Govindarajan S, Morón B, Minshull J, Khosla C. Protein engineering of improved prolyl endopeptidases for celiac sprue therapy. Protein Eng. Des. Sel. 2008 Oct 4; 21(12)699-707.
[] Besedin DV, Rudenskaya GN. Proline-Specific Endopeptidases. Russ. J. Bioorg. Chem. 2002 Feb 28; 29(1)1-17.
[] Gass J, Khosla C. Biomedicine and Diseases: Review Prolyl Endopeptidases. Cell. Mol. Life Sci. 2007; 64 345-55.
[] Shan L, Mathews II, Khosla C. Structural and mechanistic analysis of two prolyl endopeptidases: Role of interdomain dynamics in catalysis and specificity. PNAS. 2005 Mar 8; 102(10)3599-604.
[] Shan L, Marti T, Sollid LM, Gray GM, Khosla C. Comparative biochemical analysis of three bacterial prolyl endopeptidases: implications for coeliac sprue. Biochem. J. 2008; 383 311-18.
Proteopedia Page Contributors and Editors (what is this?)
Stacey Shantz, Michal Harel, Alexander Berchansky, Joel L. Sussman, Andrea Gorrell, David Canner