Sandbox 156
From Proteopedia
| Line 7: | Line 7: | ||
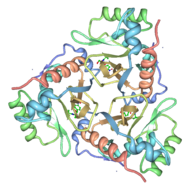
[[Image:Chloramphenicol acetyltransferase 3CLA transparent.png|thumb|190x280 px|right|Cartoon representation of CAT III]] | [[Image:Chloramphenicol acetyltransferase 3CLA transparent.png|thumb|190x280 px|right|Cartoon representation of CAT III]] | ||
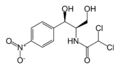
| - | Found in bacteria, the CAT III enzyme is responsible for conferring resistance of the antibiotic chloramphenicol to the cell. Chloramphenicol arrests protein synthesis by binding to the bacterial ribosomes and causing the inhibition of [http://en.wikipedia.org/wiki/Peptidyl_transferase peptidyl transferase] activity<ref>PMID: 1544895</ref>. However, when CAT III catalyzes the acetylation of chloramphenicol, the antibiotic can no longer bind to the ribosomes and is rendered inactive. The genes for the enzyme are commonly found on the plasmid of the bacteria and have been found in a numerous bacterial species<ref>PMID: 2268277</ref>. | + | Found in bacteria, the CAT III enzyme is responsible for conferring resistance of the antibiotic chloramphenicol to the cell. Chloramphenicol arrests protein synthesis by binding to the bacterial ribosomes and causing the inhibition of |
| + | [http://en.wikipedia.org/wiki/Peptidyl_transferase peptidyl transferase] activity<ref name=”1”>PMID: 1544895</ref>. However, when CAT III catalyzes the acetylation of chloramphenicol, the antibiotic can no longer bind to the ribosomes and is rendered inactive. The genes for the enzyme are commonly found on the plasmid of the bacteria and have been found in a numerous bacterial species<ref name=”2”>PMID: 2268277</ref>. | ||
| + | |||
| + | |||
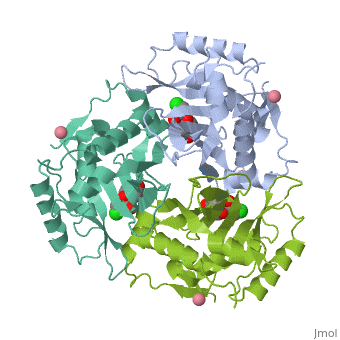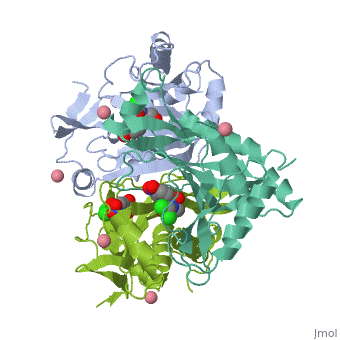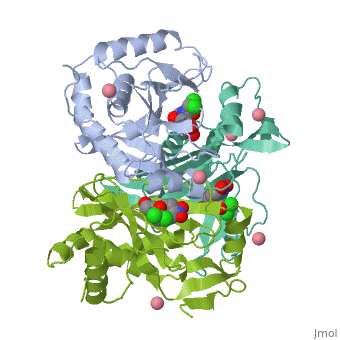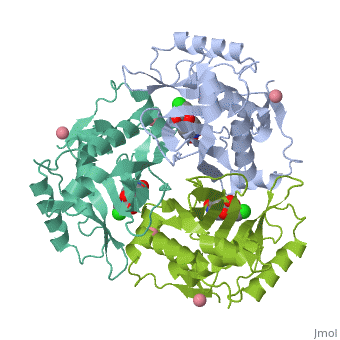
| + | The multifunctional enzyme consists of three identical subunits with three active sites at the subunit interfaces. The side chains of one subunit allow [http://en.wikipedia.org/wiki/Van_der_Waals_force van der Waals interactions] and two hydrogen bonds with chloramphenicol, causing binding of the substrate. The opposing subunit provides a histidine (His-195) residue essential for catalysis<ref>PMID: 8407936</ref>. Water molecules in the cavity provide a bridging hydrogen bond between the 1-hydroxyl of chloramphenicol and the hydroxyl of a threonine (Thr-174) residue. The active site of CAT III performs two acetylations of chloramphenicol and can accommodate the presence of the first intermediates quite well<ref name=”Murray”>PMID:2015231</ref>. | ||
| - | The multifunctional enzyme consists of three identical subunits with three active sites at the subunit interfaces. The side chains of one subunit allow [http://en.wikipedia.org/wiki/Van_der_Waals_force van der Waals interactions] and two hydrogen bonds with chloramphenicol, causing binding of the substrate. The opposing subunit provides a histidine (His-195) residue essential for catalysis<ref>PMID: 8407936</ref>. Water molecules in the cavity provide a bridging hydrogen bond between the 1-hydroxyl of chloramphenicol and the hydroxyl of a threonine (Thr-174) residue. The active site of CAT III performs two acetylations of chloramphenicol and can accommodate the presence of the first intermediates quite well<ref>PMID:2015231</ref>. | ||
=== Reaction of CAT III === | === Reaction of CAT III === | ||
| Line 17: | Line 20: | ||
[[Image:Picture2.jpg|500x400 px|center]] | [[Image:Picture2.jpg|500x400 px|center]] | ||
| - | In the first step of the reaction, Histidine-195 abstracts a proton from the 3-hydroxyl of chloramphenicol, promoting a [http://en.wikipedia.org/wiki/Nucleophilic_attack nucleophilic attack] from the [http://en.wikipedia.org/wiki/Oxyanion oxyanion] to the thioester bond of the acetyl-CoA. The intermediate produced, 3-acetylchloramphenicol, then rearranges non-enzymatically to 1-acetylchloramphenicol. Regeneration of the 3-hydroxyl allows another round of CAT III catalyzed nucleophilic attack and a 1,3-diacetylchloramphenicol product is formed<ref>PMID:2015231</ref>. | + | In the first step of the reaction, Histidine-195 abstracts a proton from the 3-hydroxyl of chloramphenicol, promoting a [http://en.wikipedia.org/wiki/Nucleophilic_attack nucleophilic attack] from the [http://en.wikipedia.org/wiki/Oxyanion oxyanion] to the thioester bond of the acetyl-CoA. The intermediate produced, 3-acetylchloramphenicol, then rearranges non-enzymatically to 1-acetylchloramphenicol. Regeneration of the 3-hydroxyl allows another round of CAT III catalyzed nucleophilic attack and a 1,3-diacetylchloramphenicol product is formed<ref name="Murray">PMID:2015231</ref>. |
==Structure== | ==Structure== | ||
| Line 26: | Line 29: | ||
===The Chloramphenicol Binding Site=== | ===The Chloramphenicol Binding Site=== | ||
| - | The association of the monomeric subunits produces a well-defined pocket at the subunit interface, allowing for the binding of the chloramphenicol molecule. The <scene name='Sandbox_156/Scene_3/1'>chloramphenicol binding site</scene> of CAT III is lined with hydrophobic residues, allowing | + | The association of the monomeric subunits produces a well-defined pocket at the subunit interface, allowing for the binding of the chloramphenicol molecule. The <scene name='Sandbox_156/Scene_3/1'>chloramphenicol binding site</scene> of CAT III is lined with hydrophobic residues, allowing |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Revision as of 18:25, 28 March 2010
Contents |
Chloramphenicol Acetyltransferase Type III
Chloramphenicol acetyltransferase type III (CAT III) is an enzyme which catalyzes the transfer of the acetyl group from acetyl-CoA to hydroxyl groups of chloramphenicol. CAT III is a trimeric protein with a Mr of 25 000-kDa and a member of the actetyltransferase family of proteins.
Introduction
Found in bacteria, the CAT III enzyme is responsible for conferring resistance of the antibiotic chloramphenicol to the cell. Chloramphenicol arrests protein synthesis by binding to the bacterial ribosomes and causing the inhibition of peptidyl transferase activity[1]. However, when CAT III catalyzes the acetylation of chloramphenicol, the antibiotic can no longer bind to the ribosomes and is rendered inactive. The genes for the enzyme are commonly found on the plasmid of the bacteria and have been found in a numerous bacterial species[2].
The multifunctional enzyme consists of three identical subunits with three active sites at the subunit interfaces. The side chains of one subunit allow van der Waals interactions and two hydrogen bonds with chloramphenicol, causing binding of the substrate. The opposing subunit provides a histidine (His-195) residue essential for catalysis[3]. Water molecules in the cavity provide a bridging hydrogen bond between the 1-hydroxyl of chloramphenicol and the hydroxyl of a threonine (Thr-174) residue. The active site of CAT III performs two acetylations of chloramphenicol and can accommodate the presence of the first intermediates quite well[4].
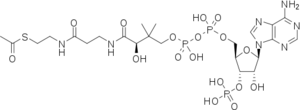
Reaction of CAT III
Both reactions take place in the active site of CAT III, where acetyl-CoA is tunneled through from the opposing side of the trimer[5].
In the first step of the reaction, Histidine-195 abstracts a proton from the 3-hydroxyl of chloramphenicol, promoting a nucleophilic attack from the oxyanion to the thioester bond of the acetyl-CoA. The intermediate produced, 3-acetylchloramphenicol, then rearranges non-enzymatically to 1-acetylchloramphenicol. Regeneration of the 3-hydroxyl allows another round of CAT III catalyzed nucleophilic attack and a 1,3-diacetylchloramphenicol product is formed[6].
Structure
|
The general structure of CAT III is dominated by a six stranded antiparallel and 5 stacked against the ends and face of the protein, forming a structure known as an "open-faced sandwhich"[7]. An extended β-strand forms an extension of the six stranded sheet to a seven stranded sheet that spans the interface of the subunit. Three identical monomers associate to form the trimeric protein with two ions acting as cofactors.
The Chloramphenicol Binding Site
The association of the monomeric subunits produces a well-defined pocket at the subunit interface, allowing for the binding of the chloramphenicol molecule. The of CAT III is lined with hydrophobic residues, allowing