Sandbox 172
From Proteopedia
| Line 1: | Line 1: | ||
{{STRUCTURE_1cza| PDB=1cza | SCENE=Sandbox_172/Mynewscene/1 }} | {{STRUCTURE_1cza| PDB=1cza | SCENE=Sandbox_172/Mynewscene/1 }} | ||
==Hexokinase Type I== | ==Hexokinase Type I== | ||
| - | + | Hexokinase Type I is one of four [http://en.wikipedia.org/wiki/Hexokinase hexokinase] isoenzymes that participate in [http://en.wikipedia.org/wiki/Glycolysis glycolysis]. Hexokinase catalyzes the phosphorylation of glucose into glucose-6-phosphate (G6P) providing enough activation energy for the glycolytic process to start. Thus hexokinase allows the muscle cells to take up glucose in the blood and use it as an energy source for different actions and expenditures. Hexokinase Type I is mainly found in mammalian tissues; it is crucial enzyme in maintaining a downward concentration gradient to provide a steady influx of glucose into cells. | |
==Introduction== | ==Introduction== | ||
Four types of hexokinase isozymes exist in the human biological system. They all serve to catalyze the exact same reaction in glycolysis even though they are encoded by different sets of genes. The hexokinase types I-III all have a high affinity for glucose and become subject to inhibition in the presence of glucose-6-phosphate- the first step reaction product in glycolysis. Hexokinase type I is the predominant form in the muscle while hexokinase type II is the predominant form in myocytes. Hexokinase type IV varies from the other three hexokinase types as it is not inhibited by the production of glucose-6-phosphate and shows a lower affinity for glucose. Hexokinase type I and II have the particular ability to bind to outer mitochondrial membrane, this binding happens both specifically and reversibly.<ref name="one">Garavito R., Mulichuk A., Padmanabhan K., Wilson J. The structure of mammalian hexokinase-1. Nature Structural and Molecular Biology. 1998. '''5''': 555-560.</ref> | Four types of hexokinase isozymes exist in the human biological system. They all serve to catalyze the exact same reaction in glycolysis even though they are encoded by different sets of genes. The hexokinase types I-III all have a high affinity for glucose and become subject to inhibition in the presence of glucose-6-phosphate- the first step reaction product in glycolysis. Hexokinase type I is the predominant form in the muscle while hexokinase type II is the predominant form in myocytes. Hexokinase type IV varies from the other three hexokinase types as it is not inhibited by the production of glucose-6-phosphate and shows a lower affinity for glucose. Hexokinase type I and II have the particular ability to bind to outer mitochondrial membrane, this binding happens both specifically and reversibly.<ref name="one">Garavito R., Mulichuk A., Padmanabhan K., Wilson J. The structure of mammalian hexokinase-1. Nature Structural and Molecular Biology. 1998. '''5''': 555-560.</ref> | ||
Revision as of 00:07, 31 March 2010
| |||||||||
| 1cza, resolution 1.90Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||||
| Activity: | Hexokinase, with EC number 2.7.1.1 | ||||||||
| Related: | 1hkb, 1hkc | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
Hexokinase Type I
Hexokinase Type I is one of four hexokinase isoenzymes that participate in glycolysis. Hexokinase catalyzes the phosphorylation of glucose into glucose-6-phosphate (G6P) providing enough activation energy for the glycolytic process to start. Thus hexokinase allows the muscle cells to take up glucose in the blood and use it as an energy source for different actions and expenditures. Hexokinase Type I is mainly found in mammalian tissues; it is crucial enzyme in maintaining a downward concentration gradient to provide a steady influx of glucose into cells.
Introduction
Four types of hexokinase isozymes exist in the human biological system. They all serve to catalyze the exact same reaction in glycolysis even though they are encoded by different sets of genes. The hexokinase types I-III all have a high affinity for glucose and become subject to inhibition in the presence of glucose-6-phosphate- the first step reaction product in glycolysis. Hexokinase type I is the predominant form in the muscle while hexokinase type II is the predominant form in myocytes. Hexokinase type IV varies from the other three hexokinase types as it is not inhibited by the production of glucose-6-phosphate and shows a lower affinity for glucose. Hexokinase type I and II have the particular ability to bind to outer mitochondrial membrane, this binding happens both specifically and reversibly.[1]
Structural Overview
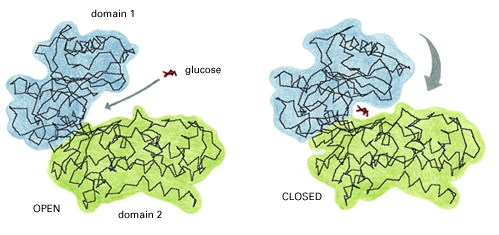
The size of hexokinase type I is approximately 100 kD.[2] Hexokinase type I is constructed by a N-terminal regulatory domain and a C-terminal catalytic domain joined together by an alpha helix. The glucose binding site of hexokinase type I can be found within the two sub-units that make up the isoenzyme, these are known as lobes. Factors that contribute to the binding of glucose to this active site include amino acids within the actual site and hydrogen bonding that takes place on the glucose between the hydroxyl groups.
Glucose Binding Sites
The residues of the glucose binding site of Hexokinase Type I are very highly conserved within the hexokinase sequence; glucose binds equally to both domains or "lobes" of the structure. As a result, hexokinase type I in its native conformation has an active site in its inactive regulatory domains. Before glucose binds to hexokinase type I, it is said to be in an open conformation. ATP is already bound within one of the domains but it is situated a distance from the glucose binding site. As the glucose binds, the two domains close around the glucose substrate and this change results in a newly formed pattern of hydrogen bonding.
Active Site
In the of hexokinase type I, Lys 621 and Asp 657 show the hydrogen-bonding distance in which a reactive O6 hydroxyl is situated in between. Here Lys 621 functions to aid the transfer of the phosphate moiety which is negatively charged; Asp 657 serves as a catalytic base or to position the glucose O6 correctly for phosphoryl transfer to take place. Though it may seem that Ser 603 may have functional significance, there has been no observed interaction of Ser 603 with glucose substrates. A torsional rotation of of the hydroxyl group brings about a much better distance for hydrogen bonding of the glucose O6. As a result of this observation, it can be assumed that Ser 603 may have some transient role during phosphorylation.[1]
Regulatory Binding Site
As glucose-6-phosphate is being produced it binds to either one of the domains on hexokinase type I. On domain 1, the phosphate moiety is surrounded by Ser 88, Thr 232, and Ser 415. The presence of these three residues generate an anion binding site that is approximately 5.6 Å to the 6 hydroxyl of the glucose that is bound.[1]
Functional Overview
Hexokinase Type I functions in a mainly catabolic role; it is responsible for introducing glucose the glycolytic process in attempts to produce ATP. Hexokinase Type I phosphorylates a hexose into a hexose phosphate; most commonly the substrate of hexokinase I is found to be glucose and the product found to be glucose-6-phosphate. Mammalian brain tissue shows a high content of Hexokinase Type I which reiterates the idea that this isoenzyme is needed to maintain high rates of energy metabolism. Hexokinase Type I associated with brain homogenates demonstration an interaction with outer mitochondrial membrane. The binding of to this mitochondria is highly dependent on the N-terminus sequence. The protein porin is then responsible for the formation of a channel in which metabolites can pass through the mitochondrial membrame.[3]
References
- ↑ 1.0 1.1 1.2 Garavito R., Mulichuk A., Padmanabhan K., Wilson J. The structure of mammalian hexokinase-1. Nature Structural and Molecular Biology. 1998. 5: 555-560.
- ↑ Fromm H., Zewe V. Kinetic studies of the brain hexokinase reaction. The Journal of Biological Chemistry. 1962.235:1661-1667.
- ↑ Wilson, J. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. The Journal of Experimental Biology. 2003.206:2049-2057.
| Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. Andrea Gorrell. |