Sandbox 160
From Proteopedia
| Line 6: | Line 6: | ||
{{STRUCTURE_1vc2| PDB=1vc2 | Scene =Sandbox_160/Newscene/1'>active site }} | {{STRUCTURE_1vc2| PDB=1vc2 | Scene =Sandbox_160/Newscene/1'>active site }} | ||
| - | Glyceraldehyde 3-Phosphate dehydrogenase (GAPDH) is an Oxidoreductase enzyme and is involved in many important biochemical reactions. It is involved in glycolysis, gluconeogenesis and in the case of photosynthetic organism, the carbon reduction cycle <ref name=" | + | Glyceraldehyde 3-Phosphate dehydrogenase (GAPDH) is an Oxidoreductase enzyme and is involved in many important biochemical reactions. GAPDH has been divided into two large classes and subsequent subclasses. Class 1 consists of eukaryotes and eubacteria whereas class 2 contains archael GAPDHs <ref name="ref 2"/> . It is involved in glycolysis, gluconeogenesis and in the case of photosynthetic organism, the carbon reduction cycle <ref name="ref 2"/>. This protein is responsible for catalyzing the conversion of glyceraldeyde 3-Phosphate into 1,3-Biphosphoglycerate in a two step coupled mechanism. This conversion occurs during step 6 or the beginning of the "payoff phase" of glycolysis (the second half of the entire process) in which ATP and NADH is produced. A total of 2 NADH and 4 ATP are produced during this phase for a net gain of 2 NADH and 2 ATP for the entire glycolysis pathway per glucose. |
== Structure & Function == | == Structure & Function == | ||
| - | Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) has carefully been studied in a number of bacterial, parasitic and mammalian species and it has been found that it exists as homotetrameric protein <ref name="reference 1"/>. Two anion binding sites have been found where the two phosphates involved in the reaction will be bound during catalysis. One site is labeled "Pi" and is the location where the inorganic phosphate involved will bind and the other has been labeled "Ps" which is where the C-3 phosphate of Gylceraldeyhde 3-Phosphate will bind <ref name="reference 1"/>. Further experimentation has shown that the "Ps" site has been conserved in numerous GAPDH complexes and the the former may involve two possible sites. | + | Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH)has carefully been studied in a number of bacterial, parasitic and mammalian species and it has been found that it exists as homotetrameric protein <ref name="reference 1"/>. Two anion binding sites have been found where the two phosphates involved in the reaction will be bound during catalysis. One site is labeled "Pi" and is the location where the inorganic phosphate involved will bind and the other has been labeled "Ps" which is where the C-3 phosphate of Gylceraldeyhde 3-Phosphate will bind <ref name="reference 1"/> . Further experimentation has shown that the "Ps" site has been conserved in numerous GAPDH complexes and the the former may involve two possible sites. |
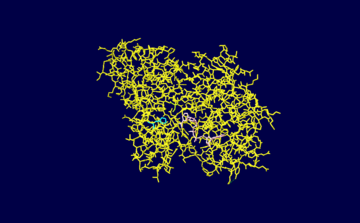
The enzyme contains a functional NAD+ group which functions as a hydrogen acceptor during the course of the reaction which is bound to a Rossman fold. During the catalysis of glyceraldehyde 3-phosphate to 1,3-biphosphoglycerate a hydride ion is enzymatically transferred from the aldehyde group of glyceraldehyde 3-phosphate to the nicotinamide ring of NAD+ reducing it to NADH (pink)<ref name="reference 1">PMID:19243605 </ref>. The active site of GAPDH contains a cysteine (Cys149 colored green) residue which reacts with the glyceraldehyde 3-phosphate molecule through its -SH group. The substrate is covalently bound during the reaction through its aldehyde group to the -SH group of the cysteine residue and the resulting reaction produces a thiohemiacetal intermediate <ref name="reference 1"/>. Note that this reaction occurs through acid base catalysis with aid of a histidine residue (His176).The <scene name='Sandbox_160/Newscene/1'>active site</scene> of the molecule is illustrated to the right. | The enzyme contains a functional NAD+ group which functions as a hydrogen acceptor during the course of the reaction which is bound to a Rossman fold. During the catalysis of glyceraldehyde 3-phosphate to 1,3-biphosphoglycerate a hydride ion is enzymatically transferred from the aldehyde group of glyceraldehyde 3-phosphate to the nicotinamide ring of NAD+ reducing it to NADH (pink)<ref name="reference 1">PMID:19243605 </ref>. The active site of GAPDH contains a cysteine (Cys149 colored green) residue which reacts with the glyceraldehyde 3-phosphate molecule through its -SH group. The substrate is covalently bound during the reaction through its aldehyde group to the -SH group of the cysteine residue and the resulting reaction produces a thiohemiacetal intermediate <ref name="reference 1"/>. Note that this reaction occurs through acid base catalysis with aid of a histidine residue (His176).The <scene name='Sandbox_160/Newscene/1'>active site</scene> of the molecule is illustrated to the right. | ||
| Line 19: | Line 19: | ||
The Pi that is involved in the reaction functions to attack by phosphorolysis the thioester intermediate that is formed by the substrate on the cysteine reside after NAD+ has been reduced <ref name="reference 1"/>. The attack by Pi on the carbonyl carbon of C1 is simultaneously followed by the replacement of bound NADH for NAD+ so another turn of the cycle can now commence. The final product is released as 1,3 bisphosphoglycerate in which the second Pi molecule has been incorporated. | The Pi that is involved in the reaction functions to attack by phosphorolysis the thioester intermediate that is formed by the substrate on the cysteine reside after NAD+ has been reduced <ref name="reference 1"/>. The attack by Pi on the carbonyl carbon of C1 is simultaneously followed by the replacement of bound NADH for NAD+ so another turn of the cycle can now commence. The final product is released as 1,3 bisphosphoglycerate in which the second Pi molecule has been incorporated. | ||
| - | <ref name=" | + | <ref name="ref 2">PMID:11846565 </ref>. |
Revision as of 00:51, 1 April 2010
Contents |
Glyceraldehyde 3-Phosphate Dehydrogenase
Introduction
| |||||||||
| 1vc2, resolution 2.60Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| Activity: | Glyceraldehyde-3-phosphate dehydrogenase (phosphorylating), with EC number 1.2.1.12 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum, TOPSAN | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Glyceraldehyde 3-Phosphate dehydrogenase (GAPDH) is an Oxidoreductase enzyme and is involved in many important biochemical reactions. GAPDH has been divided into two large classes and subsequent subclasses. Class 1 consists of eukaryotes and eubacteria whereas class 2 contains archael GAPDHs [1] . It is involved in glycolysis, gluconeogenesis and in the case of photosynthetic organism, the carbon reduction cycle [1]. This protein is responsible for catalyzing the conversion of glyceraldeyde 3-Phosphate into 1,3-Biphosphoglycerate in a two step coupled mechanism. This conversion occurs during step 6 or the beginning of the "payoff phase" of glycolysis (the second half of the entire process) in which ATP and NADH is produced. A total of 2 NADH and 4 ATP are produced during this phase for a net gain of 2 NADH and 2 ATP for the entire glycolysis pathway per glucose.
Structure & Function
Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH)has carefully been studied in a number of bacterial, parasitic and mammalian species and it has been found that it exists as homotetrameric protein [2]. Two anion binding sites have been found where the two phosphates involved in the reaction will be bound during catalysis. One site is labeled "Pi" and is the location where the inorganic phosphate involved will bind and the other has been labeled "Ps" which is where the C-3 phosphate of Gylceraldeyhde 3-Phosphate will bind [2] . Further experimentation has shown that the "Ps" site has been conserved in numerous GAPDH complexes and the the former may involve two possible sites.
The enzyme contains a functional NAD+ group which functions as a hydrogen acceptor during the course of the reaction which is bound to a Rossman fold. During the catalysis of glyceraldehyde 3-phosphate to 1,3-biphosphoglycerate a hydride ion is enzymatically transferred from the aldehyde group of glyceraldehyde 3-phosphate to the nicotinamide ring of NAD+ reducing it to NADH (pink)[2]. The active site of GAPDH contains a cysteine (Cys149 colored green) residue which reacts with the glyceraldehyde 3-phosphate molecule through its -SH group. The substrate is covalently bound during the reaction through its aldehyde group to the -SH group of the cysteine residue and the resulting reaction produces a thiohemiacetal intermediate [2]. Note that this reaction occurs through acid base catalysis with aid of a histidine residue (His176).The of the molecule is illustrated to the right.
A simplified illustration of the net reaction is as follows:
D-glyceraldehyde 3-phosphate + phosphate + NAD+ ---------> 1,3 biphospho-D-glycerate + NADH + H+
The Pi that is involved in the reaction functions to attack by phosphorolysis the thioester intermediate that is formed by the substrate on the cysteine reside after NAD+ has been reduced [2]. The attack by Pi on the carbonyl carbon of C1 is simultaneously followed by the replacement of bound NADH for NAD+ so another turn of the cycle can now commence. The final product is released as 1,3 bisphosphoglycerate in which the second Pi molecule has been incorporated.
[1].
Active Site in Detail
References
- ↑ 1.0 1.1 1.2 Fermani S, Ripamonti A, Sabatino P, Zanotti G, Scagliarini S, Sparla F, Trost P, Pupillo P. Crystal structure of the non-regulatory A(4 )isoform of spinach chloroplast glyceraldehyde-3-phosphate dehydrogenase complexed with NADP. J Mol Biol. 2001 Nov 30;314(3):527-42. PMID:11846565 doi:10.1006/jmbi.2001.5172
- ↑ 2.0 2.1 2.2 2.3 2.4 Cook WJ, Senkovich O, Chattopadhyay D. An unexpected phosphate binding site in glyceraldehyde 3-phosphate dehydrogenase: crystal structures of apo, holo and ternary complex of Cryptosporidium parvum enzyme. BMC Struct Biol. 2009 Feb 25;9:9. PMID:19243605 doi:10.1186/1472-6807-9-9